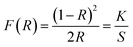Nonlinear optical response mechanism of noncentrosymmetric lead borate Pb6[B4O7(OH)2]3 with three crystallographically independent [B4O7(OH)2]4− chains†
Xuefei Wangab,
Fangfang Zhang*a,
Bing-Hua Leiab,
Zhihua Yanga and
Shilie Pan*a
aKey Laboratory of Functional Materials and Devices for Special Environments, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Xinjiang Key Laboratory of Electronic Information Materials and Devices, 40-1 South Beijing Road, Urumqi 830011, China. E-mail: ffzhang@ms.xjb.ac.cn; slpan@ms.xjb.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 17th October 2016
Abstract
The noncentrosymmetric lead borate Pb6[B4O7(OH)2]3 has been synthesized by hydrothermal method with a yield of ∼85% based on Pb. It structurally features three crystallographically independent [B4O7(OH)2]4− helical chains with B12O29 fundamental building blocks (FBBs), which is unique in borate system. It has a wide transparency range from UV to NIR with the shortwave cut-off edge about 260 nm. The phase-matching second harmonic generation (SHG) response at 1064 nm fundamental wave was identified by the Kurtz–Perry method. First principles calculations on the band structure and partial density of states (PDOS) show that the title compound has a direct band gap determined by the interaction between lead and oxygen atoms. Stereochemically active lone pair lead cations and the BO3 groups with π-conjugated system make main contribution to the nonlinear optical (NLO) effect according to the SHG-density method.
Introduction
Borates with varied structures have attracted a great deal of attention over the past few decades.1 Unlike the regular coordination of carbon, nitrogen and phosphorus atoms in carbonates, nitrates and phosphates, the boron atoms in borates can coordinate with either three or four oxygen atoms to form [BO3]3− or [BO4]5− anionic polyhedra, and both polyhedra exist as isolated clusters, or connect with each other via sharing corners or edges resulting in a variety of one-dimensional chains, two-dimensional layers, or three-dimensional frameworks.2 In the process of exploring new materials with functional properties, borates are usually regarded as good candidates for nonlinear optical (NLO) materials due to the following merits: (i) the ratio of noncentrosymmetric (NCS) structures (the prerequisite for SHG effect) in borates is much higher than that of all inorganic compounds. Our statistical analysis on the structural data in the Inorganic Crystal Structure Database (ICSD, 2016-5, Version 3.4.0, by Fachinformationszentrum Karlsruhe) shows that ∼40.5% of known borates possess NCS structures while the ratio is ∼17.8% for all inorganic compounds; (ii) a series of advantages, such as wide transparency range, high damage threshold and good thermo-stability, also make borates be desired NLO materials.3 Several borates with excellent NLO properties, such as β-BaB2O4 (BBO),4 LiB3O5 (LBO),5 CsB3O5 (CBO)6 and KBe2BO3F2 (KBBF),7 have been reported.It has been demonstrated that the introduction of second-order Jahn–Teller distorted cations such as stereochemically active lone pair cations (e.g., Pb2+, Bi3+, Te4+ and Se4+)8 or high valence transition-metal d0 cations (e.g., V5+, Nb5+ and Mo6+)9 is an effective strategy to enhance the SHG response. Accordingly, many compounds with excellent SHG responses such as Cd4BiO(BO3)3 (ref. 8i) and K(VO2)2O2(IO3)3 (ref. 9b) have been synthesized. Another consequence of second-order Jahn–Teller distortions is that the compounds with these cations can lead to many diverse interesting structures, such as Cs(TiOF)3(SeO3)2 (ref. 10) with interesting hexagonal tungsten oxide and Cs4Mo5P2O22 (ref. 11) with unusual chains constructed from [Mo5P2O23]6− units. Specifically, by introducing lead cations into borates, several lead(II) borates with both large SHG responses and extraordinary structures have been reported, such as PbB4O7 (ref. 12) with a framework of corner-linked BO4 tetrahedra and PbO4(BO3)2 (ref. 13) that has (Pb4O9)O(Pb4O9) units constructed by 3, 4 and 5 coordinated lead cations.
Hydrothermal reaction has been regarded as an efficient approach to the exploration of lead borates. In most cases, lead borates synthesized by hydrothermal method contain crystal water or hydroxyl groups. (PbO)3(B2O3)5(H2O)2 (ref. 14) reported by Grube in 1981, was the first lead borate synthesized by hydrothermal method. Afterwards, hydrothermal conditions were adjusted in various ways to synthesize new lead borates. Several acentric lead borates, such as Pb5(B3O8OH)3·H2O and Pb3(OH)(B9O16)B(OH)3 were obtained in high-temperature, high-pressure conditions (T ≥ 250 °C, P ≥ 70 bar).15 While in low-temperature hydrothermal conditions (T ≤ 200 °C, spontaneous pressure), strong alkali or organics (e.g. NaOH, pyridine and ethanediamine) were introduced as mineralizers, which also led to some new lead borates with SHG responses like (Pb4O)Pb2B6O14 and Pb2B3O5.5(OH)2.16
From a practical perspective, temperate reaction conditions with neutral mineralizers are more conductive. In the current work, the PbO–H3BO3–NaCl system was investigated with the low-temperature hydrothermal method and Pb6[B4O7(OH)2]3 was obtained. It was first reported by Chen et al. in 2002.17 Very recently, Huppertz et al. synthesized the compound in the Pb(BO2)2·H2O–KNO3/NaNO3–KOH hydrothermal system, and the structure re-examined accurately.18
Here in this work, Pb6[B4O7(OH)2]3 was synthesized with neutral mineralizers in a yield of ∼85% based on Pb by hydrothermal method. The structural characters of the unique [B4O7(OH)2]4− helical chains in the structure have been highlighted by comparing with other chains composed by six-membered rings. The phase-matching NLO property and the transparency range which are important for the application of NLO materials were characterized for the first time. In addition, first principles calculations on the band structure and partial density of states (PDOS) have been employed to explain their contributions to the energy band. The origins of the SHG response of Pb6[B4O7(OH)2]3 have been analysed by the SHG-density method.
Experiment section
Materials and synthesis
PbO (Tianjin Baishi Chemical Industry Co., Ltd., 99.0%), H3BO3 (Tianjin Baishi Chemical Industry Co., Ltd., 99.5%), NaCl (Tianjin Baishi Chemical Industry Co., Ltd., 99.5%) and distilled water were gathered via commercial sources, and no further purification had been done before used. The title compound was prepared by the pouch method.19 A mixture of PbO (1 mmol, 0.223 g), H3BO3 (2.4 mmol, 0.154 g), and NaCl (0.8 mmol, 0.047 g) was placed in a 5 mL heat-sealed FEP Teflon pouch. Four pouches were placed in a 75 mL Teflon-lined autoclave filled with 30 mL distilled water in order to obtain enough products under identical reaction conditions. The autoclave was closed, heated at 210 °C for 4 days and cooled to 40 °C at a rate of 2 °C per hour. Rod-like crystals of Pb6[B4O7(OH)2]3 (see Fig. S1, ESI†) were recovered with a yield of ∼85% based on Pb.Characterization
The UV-Vis-NIR diffuse reflectance spectrum measurement was carried out with polycrystalline powder of Pb6[B4O7(OH)2]3 by a Shimadzu SolidSpec-3700DUV spectrophotometer from 190 to 2600 nm in air atmosphere. The reflectance is converted to absorbance with the Kubelka–Munk function.20 The SHG response of Pb6[B4O7(OH)2]3 was measured by a modified Kurtz NLO system, which matches a Q-switched Nd:YAG laser generating 1064 nm fundamental wave.21 The polycrystalline powder of Pb6[B4O7(OH)2]3 was pulverized and separated according to distinct particle size ranges: <20, 20–38, 38–55, 55–88, 88–105, 105–150, and 150–200 μm. The SHG response of KH2PO4 (KDP) was measured as a reference for Pb6[B4O7(OH)2]3. Thermogravimetric (TG) analysis and differential scanning calorimetry (DSC) curves of Pb6[B4O7(OH)2]3 were measured by a simultaneous NETZSCH STA 449F3 thermal analyzer instrument under a flowing nitrogen atmosphere. The sample was placed in a platinum crucible, and heated from room temperature to 1000 °C at a rate of 10 °C min−1. Single crystal XRD, powder XRD, and IR spectrum measurements were performed to verify the structure and related methodologies are given in the ESI.† Relevant crystallographic data, atomic coordinates and thermal parameters, as well as selected bond lengths and angles for Pb6[B4O7(OH)2]3 are given in Tables S1–S3 in the ESI,† respectively.Computational descriptions
Single-crystal structure data of Pb6[B4O7(OH)2]3 obtained from experiments were used as origin data for theoretical calculations. Methods based on plane-wave basis set and pseudopotentials within density functional theory (DFT) from CASTAP package were employed to calculate the electronic structure and physical properties.22 The generalized gradient approximation (GGA) was processed by Perdew–Burke–Ernzerhof (PBE)23 and norm-conversing pseudopotential (NCP)24 was chosen as the functional and pseudopotential. In the computation, the following valence-electron structures were considered: Pb-5d106s26p2, B-2s22p1, O-2s22p4, and H-1s1. Giving consideration to both a relatively small plane-wave basis set and accuracy that this study required, the cut-off energy was set as 380 eV and Monkhorst–Pack k-point sampling of 2 × 2 × 2 was chosen for the numerical integration in the Brillouin zone.25 The geometry optimization was carried out on the basis of Broyden–Fletcher–Goldfarb–Shanno (BFGS) minimization technique. The converged criteria were that the residual forces on the atoms were less than 0.01 eV Å−1, the displacements of atoms were less than 5 × 10−4 Å, and the energy change was less than 5.0 × 10−6 eV per atom. The CASTEP code on the aspect of the other calculation parameters and convergent criteria were kept the default values.The “scissors” correction approximation for calculating NLO property was employed to correct the band gap. The momentum matrix elements should be modified in the scissors operator.26 The correlative renormalization of the moment matrix elements under the gap correction is satisfied,27
| Pnm → Pnm(ωnm + Δ/ħ(δnc − δmc))/ωnm |
The SHG tensors were estimated by the length-gauge formalism with a zero frequency limit.29 The total SHG coefficient χ(2) consists of two parts of contributions, namely, the processes of virtual-electron (VE) transitions and virtual-hole (VH) transitions. The static second-order coefficients can be written as
| χ(2)αβγ = χ(2)αβγ(VE) + χ(2)αβγ(VH) |
VE and VH can be calculated by the following formulas:
where α, β and γ are Cartesian components, v and v′ represent valence bands, c and c′ denote to conduction bands, and P(αβγ) refers to full permutation. The band energy difference and momentum matrix elements are respectively represented as ħωij and Pijα. More details on the calculated method were shown in ref. 29b.
The SHG-density method was employed to distinguish the contribution of the electronic states to the second-order susceptibility. Only the SHG-relevant quantum states that can take effect on the sources of SHG in real space are presented in the occupied and unoccupied SHG-density.30 On the basis of this analysis, the total SHG coefficient can be regarded as an assembly of each partial SHG coefficient in occupied and unoccupied states so that the origins of SHG can be clearly observed in real space.
Results and discussion
Crystal structure
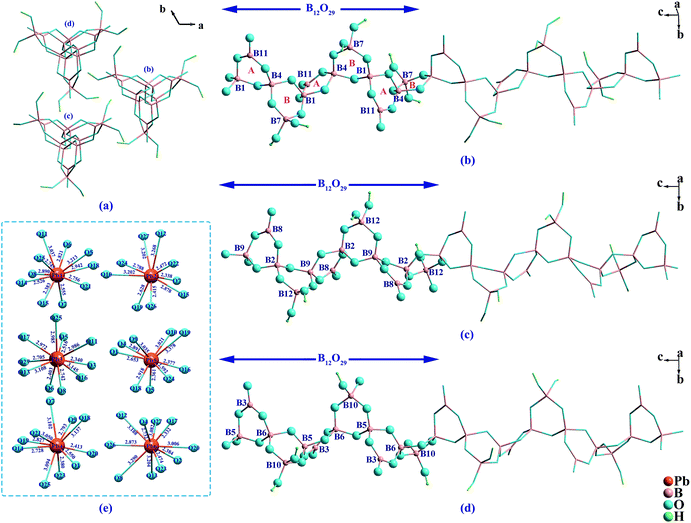
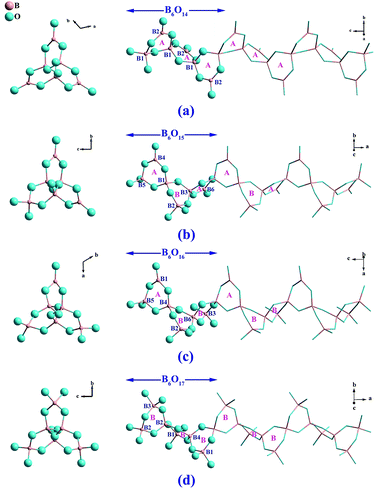
Pb6[B4O7(OH)2]3 crystallizes in the NCS group P32 of the trigonal system. As is shown in Fig. 1a, there are three crystallographically independent [B4O7(OH)2]4− helical chains with a three-fold screw axis as the central axis in the structure, which can be written as 4: ∞[(1Δ + 3T)] as introduced by Christ and Clark.31 Each [B4O7(OH)2]4− chain is composed by the fundamental building block (FBB) of B12O29 respectively. And these FBBs contain two kinds of six-membered rings named as A and B ring, respectively, where A ring contains two BO4 units and a BO3 unit, and B ring contains three BO4 units (see Fig. 1b–d). Six lead cations with nine or ten coordination environments (see Fig. 1e) connect the [B4O7(OH)2]4− chains and form the final 3D framework of Pb6[B4O7(OH)2]3. It is noticed that these Pb atoms show clear heterogeneity in their coordination environments, which can be observed from the distribution of Pb–O bonds. Each Pb atom is coordinated with surrounding O atoms with three one-sided shorter Pb–O bonds (2.3–2.6 Å) and other longer Pb–O bonds (2.6–3.4 Å). The result of bond valence sum (BVS)32 gave values of 2.0 to 2.2 for six Pb atoms. And these longer Pb–O bonds cannot be ignored otherwise the bond valences of Pb atoms are deflected. It indicates that the weak effects from oxygen atoms need to be considered for the Pb atoms in BVS calculations. The result of BVS give values of 2.8 to 3.1 for twelve B atoms and 1.8 to 2.2 for the O atoms, respectively, except O4, O8, O18, O19, O25 and O27 whose values are at 1.1 to 1.2, which can be considered as hydroxyl oxygens. Geometric method was carried out for inferring the coordinates of hydrogen atoms with coordination environments and appropriate hydrogen bond acceptors were taken into account.In borate system, chains composed by six-membered rings were found in several compounds. These compounds can be grouped into the following four categories according to their FBBs. As is shown in Fig. 2a, the chains in (Li5.5Fe0.5)FeMB12O24 (M = Ca, Sr, Ba and Pb) are formed by the FBB of B6O14 consisting of all A rings, which show that the BO3 units occupy the ratio of 1/2 of the borate units.33 While in Me3B6O11(OH)2 (Me = Sr and Ba) and SmB6O8(OH)5(B(OH)3), the B6O15 FBBs are built by both A and B rings in the sequence of -ABA-ABA-.34 Compared with the B6O14 FBB above, B6O15 with 1/3 BO3 units can be regarded as replacing a BO3 unit of B6O14 by a BO4 unit. Analogously, the B6O16 FBB in Bi3B6O13(OH) with -ABB-ABB- arrangement contains 1/6 BO3 units, and can be regarded as the further replacement between the BO3 and BO4 units (see Fig. 2c). The B6O17 FBB in Ba3B6O9(OH)6 consists of all B rings, which is a complete BO4 replacement compared with above structures. In Pb6[B4O7(OH)2]3, six-membered rings arrange in the order of -AB-AB- with 1/4 BO3 units, which is a new type of arrangement in the chains composed by six-membered rings. Considering the translational symmetry, the B12O29 FBB contains twelve B atoms are twice as long as the FBBs in above compounds.
Most of the known chain-containing borates are constructed by a chain that located in one crystallographic position. However, in Bi[B4O6(OH)2]OH, two crystallographically independent [B4O8]4− chains were observed (see Fig. S3, ESI†).8a While in Pb6[B4O7(OH)2]3, there are three crystallographically independent [B4O7(OH)2]4− chains. To the best of our knowledge, none of similar crystallographically independent chains have been reported in borate system except these two compounds, which is indicated that the structure diversity of these two compounds is caused by the flexible coordination of bismuth and lead cations.
UV-Vis-NIR diffuse reflectance spectroscopy
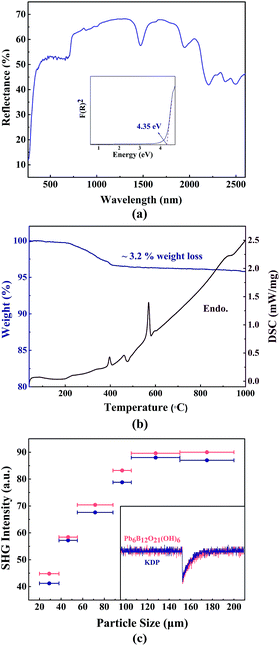
The UV-Vis-NIR diffuse reflectance spectrum of Pb6[B4O7(OH)2]3 was shown in Fig. 3a. It can be observed that the title compound embodies considerable transparency from 365 to 2600 nm with a shortwave cut-off about 260 nm. The absorption (K/S) data were transformed by Kubelka–Munk function,where R, K and S represent reflectance, absorption and scattering, respectively. The results were represented in the inset, and an approximate band gap of 4.35 eV was estimated.
Thermal analysis
TG analysis and DSC measurements were carried out for investigating the thermal property of Pb6[B4O7(OH)2]3, and the results are presented in Fig. 3b. It can be observed from the DSC curve that there is an endothermic peak at about 395 °C matched with the weight loss of 3.2%. It indicates that there are 3 water molecules are released from per formula unit of Pb6[B4O7(OH)2]3 because of the existence of hydroxyls. This result is close to the calculated theoretical weight loss of 2.98%. When heated to 460 °C, the samples that have lost weight transformed to other phases with a mitigating peak in the DSC curve. The strongest peak is shown at 570 °C, which is speculated corresponding to melting temperature.SHG measurement
Powder SHG response measurements of Pb6[B4O7(OH)2]3 and KDP were carried out, respectively. Stable green-light outputs were observed from the samples that placed under 1064 nm laser beam. It is clearly noticed that the SHG response of Pb6[B4O7(OH)2]3 is slightly higher than that of KDP from the comparison in Fig. 3c, which accords with the result from Chen et al. Specifically, phase-matching property is very important for the application of NLO crystals. Pb6[B4O7(OH)2]3 and KDP were separated into different particle size for the further measurement, respectively. And the existence of the rising-maintaining trend of the response of Pb6[B4O7(OH)2]3 indicates that it is phase-matchable according to the Kurtz–Perry method.22 The inset is oscilloscope traces of the SHG signal for the powders with particle size of 105–150 μm, which indicates that the stable green-light output of Pb6[B4O7(OH)2]3 is slightly superior to KDP.Electronic structure and NLO property analyses
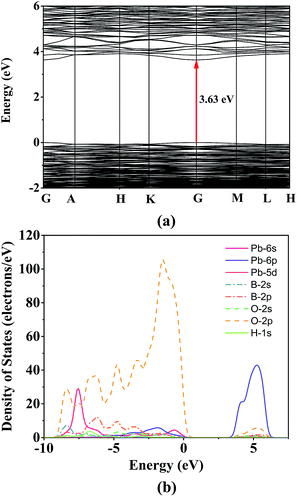
The first principles calculations were carried out for investigating the relationship between electronic structure and optical properties. The band structures of Pb6[B4O7(OH)2]3 along high-symmetry points in first Brillouin zone are presented in Fig. 4a. It can be clearly seen that the highest valence band (VB) and the lowest conduction band (CB) are both located at the G point, which indicates that Pb6[B4O7(OH)2]3 has a direct band gap with 3.63 eV. The calculated band is smaller than the experimental result (4.35 eV) due to the discontinuous exchange–correlation energy used in calculations.35 Herein, with the consideration of the underestimate in energy band gap of GGA-PBE, a scissor operator of 0.7 eV was employed while calculating the NLO properties.The PDOS of Pb6[B4O7(OH)2]3 in the region of −10 to 7.5 eV are presented in Fig. 4b. It is clearly shown that the range of −10 to −5 eV is mainly occupied by O-2p, Pb-6s orbitals, and slightly by B-2s2p and H-1s orbitals. The O-2p orbital hybridized with Pb-6s6p, O-2s and B-2s2p orbitals in the range of −5 eV to the Fermi level (set to zero eV) indicates the strong covalent interactions among lead, boron and oxygen atoms. Meanwhile, large Pb-6p orbital with a small amount of O-2s2p and Pb-6s orbital is contributed to the bottom of conduction band. Therefore, the interaction between lead and oxygen determines the band gap of Pb6[B4O7(OH)2]3.
Considered the group symmetry and Kleinman symmetry of point group of 3, four non-zero NLO coefficients, namely, d11, d22, d33 and d15 were calculated. The calculated values of d11, d22, d33 and d15 are 2.33, −0.09, 0.28 and 0.24 pm V−1, respectively. According to the phase-matching condition and effective SHG coefficients, d15 makes main contribution to the effective SHG coefficients and is in accordance with the experimental powder SHG effect.
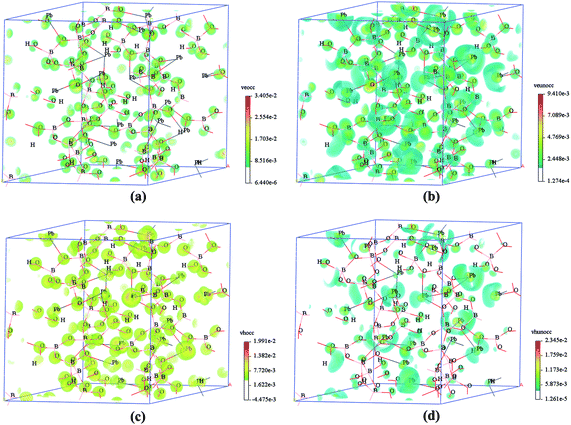
In order to get insight into the SHG response mechanism of electronic states, the SHG-density method was employed to highlight the electronic states which have SHG response, and the results were shown in Fig. 5. The SHG response of Pb6[B4O7(OH)2]3 is resolved on each electronic state for the VE and VH transitions. Both of the VE and VH processes were analysed considering the comparable contributions of them to the SHG coefficient d15. It is clearly shown that the SHG densities mainly distribute among the Pb, O and B atoms both in the occupied states and the unoccupied states. It indicates that the lead cations and the BO3 groups are the main contributors to the SHG effect because of the stereochemically active lone pair electrons in lead which have discussed before and π-conjugated interaction in BO3 groups, respectively.
Conclusions
Pb6[B4O7(OH)2]3 was successfully synthesized by low-temperature hydrothermal reactions. It crystallizes in the trigonal space group, P32, with three crystallographically independent [B4O9]6− helical chains which are connected by 9–10 coordinated Pb2+ cations. The powder SHG measurement shows that its SHG response is slightly higher than that of KDP and is phase-matchable. UV-Vis-NIR diffuse reflectance spectrum shows that its UV cut-off edge is about 260 nm. TG-DSC analysis indicates a good thermal stability up to 395 °C. First principles calculations show that it has a direct band gap determined by the interaction between lead and oxygen atoms. According to the SHG-density method, the lead cations and the BO3 groups are the main contributors to the SHG effect due to the stereochemically active lone pair electrons in lead and π-conjugated interaction in the BO3 groups, respectively. We believe that our work can give important guidance on further NLO study in lead borates.Acknowledgements
The authors acknowledge the financial support by the West Light Foundation of CAS (Grant No. ZDXM-2014-01), the Special Fund for Xinjiang Key Laboratories (Grant No. 2014KL009), the Science and Technology Project of Urumqi (Grant No. P141010005).Notes and references
- (a) P. Becker, Adv. Mater., 1998, 10, 979–992 CrossRef CAS; (b) P. S. Halasyamani and K. R. Poeppelmeier, Chem. Mater., 1998, 10, 2753–2769 CrossRef CAS; (c) C. T. Chen, N. Ye, J. Lin, J. Jiang, W. R. Zeng and B. C. Wu, Adv. Mater., 1999, 11, 1071–1078 CrossRef CAS; (d) K. M. Ok, E. O. Chi and P. S. Halasyamani, Chem. Soc. Rev., 2006, 35, 710–717 RSC; (e) B. Zhang, M. H. Lee, Z. Yang, Q. Jing, S. Pan, M. Zhang, H. Wu, X. Su and C. S. Li, Appl. Phys. Lett., 2015, 106, 031906 CrossRef; (f) M. Zhang, S. L. Pan, X. Y. Fan, Z. X. Zhou, K. R. Poeppelmeier and Y. Yang, CrystEngComm, 2011, 13, 2899–2903 RSC; (g) Y. Wang and S. Pan, Coord. Chem. Rev., 2016, 323, 15–35 CrossRef CAS.
- (a) H. Huang, J. Yao, Z. Lin, X. Wang, R. He, W. Yao, N. Zhai and C. Chen, Angew. Chem., Int. Ed., 2011, 50, 9141–9144 CrossRef CAS PubMed; (b) G. Zou, N. Ye, L. Huang and X. Lin, J. Am. Chem. Soc., 2011, 133, 20001–20007 CrossRef CAS PubMed; (c) S. Zhao, P. Gong, S. Luo, L. Bai, Z. Lin, C. Ji, T. Chen, M. Hong and J. Luo, J. Am. Chem. Soc., 2014, 136, 8560–8563 CrossRef CAS PubMed; (d) J. L. Song, C. L. Hu, X. Xu, F. Kong and J. G. Mao, Angew. Chem., Int. Ed., 2015, 54, 3679–3682 CrossRef CAS PubMed; (e) L. Wang, S. Pan, L. Chang, J. Hu and H. Yu, Inorg. Chem., 2012, 51, 1852–1858 CrossRef CAS PubMed; (f) X. Fan, S. Pan, X. Hou, X. Tian, J. Han, J. Haag and K. R. Poeppelmeier, Cryst. Growth Des., 2010, 10, 252–256 CrossRef CAS; (g) Y. Yang, S. Pan, J. Han, X. Hou, Z. Zhou, W. Zhao, Z. Chen and M. Zhang, Cryst. Growth Des., 2011, 11, 3912–3916 CrossRef CAS; (h) L. Kang, M. Zhou, J. Yao, Z. Lin, Y. Wu and C. Chen, J. Am. Chem. Soc., 2015, 137, 13049–13059 CrossRef CAS PubMed.
- (a) Y. Zhou, Y. Yue, J. Wang, F. Yang, X. Cheng, D. Cui, Q. Peng, Z. Hu and Z. Xu, Opt. Express, 2009, 17, 20033–20038 CrossRef CAS PubMed; (b) S. Zhao, L. Kang, Y. Shen, X. Wang, M. A. Asghar, Z. Lin, Y. Xu, S. Zeng, M. Hong and J. Luo, J. Am. Chem. Soc., 2016, 138, 2961–2964 CrossRef CAS PubMed; (c) H. Wu, H. Yu, Z. Yang, X. Hou, X. Su, S. Pan, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2013, 135, 4215–4218 CrossRef CAS PubMed; (d) H. Wu, S. Pan, K. R. Poeppelmeier, H. Li, D. Jia, Z. Chen, X. Fan, Y. Yang, J. M. Rondinelli and H. Luo, J. Am. Chem. Soc., 2011, 133, 7786–7790 CrossRef CAS PubMed; (e) H. Wu, H. Yu, S. Pan, Z. Huang, Z. Yang, X. Su and K. R. Poeppelmeier, Angew. Chem., Int. Ed., 2013, 52, 3406–3410 CrossRef CAS PubMed; (f) C. Bai, S. Han, S. Pan, B. Zhang, Y. Yang, L. Li and Z. Yang, RSC Adv., 2015, 5, 12416–12422 RSC.
- C. T. Chen, B. C. Wu, A. D. Jiang and G. M. You, Sci. Sin., Ser. B, 1985, 28, 235–243 Search PubMed.
- C. Chen, Y. Wu, A. Jiang, B. Wu, G. You, R. Li and S. Lin, J. Opt. Soc. Am. B, 1989, 6, 616–621 CrossRef CAS.
- Y. C. Wu, T. Sasaki, S. Nakai, A. Yokotani, H. G. Tang and C. T. Chen, Appl. Phys. Lett., 1993, 62, 2614–2615 CrossRef CAS.
- C. T. Chen, Y. B. Wang, B. C. Wu, K. C. Wu, W. L. Zeng and L. H. Yu, Nature, 1995, 373, 322–324 CrossRef CAS.
- (a) L. Li, G. Li, Y. Wang, F. Liao and J. Lin, Chem. Mater., 2005, 17, 4174–4180 CrossRef CAS; (b) H. Li, H. Wu, X. Su, H. Yu, S. Pan, Z. Yang, Y. Lu, J. Han and K. R. Poeppelmeier, J. Mater. Chem. C, 2014, 2, 1704–1710 RSC; (c) F. Li, X. Hou, S. Pan and X. Wang, Chem. Mater., 2009, 21, 2846–2850 CrossRef CAS; (d) F. Li, S. Pan, X. Hou and J. Yao, Cryst. Growth Des., 2009, 9, 4091–4095 CrossRef CAS; (e) H. Yu, H. Wu, Q. Jing, Z. Yang, P. S. Halasyamani and S. Pan, Chem. Mater., 2015, 27, 4779–4788 CrossRef CAS; (f) H. Zhang, M. Zhang, S. Pan, X. Dong, Z. Yang, X. Hou, Z. Wang, K. B. Chang and K. R. Poeppelmeier, J. Am. Chem. Soc., 2015, 137, 8360–8363 CrossRef CAS PubMed; (g) X. Dong, Q. Jing, Y. Shi, Z. Yang, S. Pan, K. R. Poeppelmeier, J. Young and J. M. Rondinelli, J. Am. Chem. Soc., 2015, 137, 9417–9422 CrossRef CAS PubMed; (h) G. Zou, G. Nam, H. G. Kim, H. Jo, T. S. You and K. M. Ok, RSC Adv., 2015, 5, 84754–84761 RSC; (i) W. Zhang, W. Chen, H. Zhang, L. Geng, C. Lin and Z. He, J. Am. Chem. Soc., 2010, 132, 1508–1509 CrossRef CAS PubMed; (j) F. Kong, S. P. Huang, Z. M. Sun, J. G. Mao and W. D. Cheng, J. Am. Chem. Soc., 2006, 128, 7750–7751 CrossRef CAS PubMed; (k) S. Y. Song and K. M. Ok, Inorg. Chem., 2015, 54, 5032–5038 CrossRef CAS PubMed.
- (a) P. A. Maggard, C. L. Stern and K. R. Poeppelmeier, J. Am. Chem. Soc., 2001, 123, 7742–7743 CrossRef CAS PubMed; (b) C. F. Sun, C. L. Hu, X. Xu, B. P. Yang and J. G. Mao, J. Am. Chem. Soc., 2011, 133, 5561–5572 CrossRef CAS PubMed; (c) M. D. Donakowski, R. Gautier, J. Yeon, D. T. Moore, J. C. Nino, P. S. Halasyamani and K. R. Poeppelmeier, J. Am. Chem. Soc., 2012, 134, 7679–7689 CrossRef CAS PubMed; (d) H. Yu, H. Wu, S. Pan, Z. Yang, X. Hou, X. Su, Q. Jing, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2014, 136, 1264–1267 CrossRef CAS PubMed; (e) Y. Shi, S. Pan, X. Dong, Y. Wang, M. Zhang, F. Zhang and Z. Zhou, Inorg. Chem., 2012, 51, 10870–10875 CrossRef CAS PubMed; (f) S. E. Bang and K. M. Ok, Inorg. Chem., 2015, 54, 8832–8839 CrossRef CAS PubMed; (g) S. Luo, L. Kang, Z. Lin and C. Chen, RSC Adv., 2014, 4, 27122–27125 RSC.
- X. L. Cao, C. L. Hu, F. Kong and J. G. Mao, Inorg. Chem., 2015, 54, 3875–3882 CrossRef CAS PubMed.
- Y. Wang, S. Pan, H. Yu, X. Su, M. Zhang, F. Zhang and J. Han, Chem. Commun., 2013, 49, 306–308 RSC.
- L. Bohatý, Z. Kristallogr. - Cryst. Mater., 1983, 164, 279–283 Search PubMed.
- H. Yu, S. Pan, H. Wu, W. Zhao, F. Zhang, H. Li and Z. Yang, J. Mater. Chem., 2012, 22, 2105–2110 RSC.
- H. H. Grube, Fortschr. Mineral., 1981, 59, 58–59 Search PubMed.
- (a) R. K. Rastsvetaeva, A. V. Arakcheeva, D. Y. Pushcharovsky, S. A. Vinogradova, O. V. Dimitrova and S. Y. Stefanovich, Z. Kristallogr. - Cryst. Mater., 1998, 213, 240–245 CAS; (b) E. L. Belokoneva, S. Y. Stefanovich, T. A. Borisova and O. V. Dimitrova, Russ. J. Inorg. Chem., 2001, 46, 1621–1627 Search PubMed.
- (a) J. L. Song, C. L. Hu, X. Xu, F. Kong and J. G. Mao, Inorg. Chem., 2013, 52, 8979–8986 CrossRef CAS PubMed; (b) F. Zhang, F. Zhang, B. H. Lei, Z. Yang and S. Pan, J. Phys. Chem. C, 2016, 120, 12757–12764 CrossRef CAS.
- Z. T. Yu, Z. Shi, Y. S. Jiang, H. M. Yuan and J. S. Chen, Chem. Mater., 2002, 14, 1314–1318 CrossRef CAS.
- S. Schönegger, S. Ortner Teresa, K. Wurst, G. Heymann and H. Huppertz, Z. Naturforsch., B: J. Chem. Sci., 2016, 71, 925–933 Search PubMed.
- (a) W. T. A. Harrison, T. M. Nenoff, T. E. Gier and G. D. Stucky, Inorg. Chem., 1993, 32, 2437–2441 CrossRef CAS; (b) S. Allen, S. Barlow, P. S. Halasyamani, J. F. W. Mosselmans, D. O'Hare, S. M. Walker and R. I. Walton, Inorg. Chem., 2000, 39, 3791–3798 CrossRef CAS PubMed; (c) B. J. Ingram, G. B. González, T. O. Mason, D. Y. Shahriari, A. Barnabè, D. Ko and K. R. Poeppelmeier, Chem. Mater., 2004, 16, 5616–5622 CrossRef CAS; (d) J. M. Chamberlain, T. A. Albrecht, J. Lesage, F. Sauvage, C. L. Stern and K. R. Poeppelmeier, Cryst. Growth Des., 2010, 10, 4868–4873 CrossRef CAS.
- P. Kubelka and F. Munk, Z. Tech. Phys., 1931, 12, 593–604 Search PubMed.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr., 2005, 220, 567–570 CAS.
- A. M. Rappe, K. M. Rabe, E. Kaxiras and J. D. Joannopoulos, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 1227–1230 CrossRef.
- (a) J. S. Lin, A. Qteish, M. C. Payne and V. Heine, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 4174–4180 CrossRef; (b) J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 5188–5192 CrossRef.
- (a) R. W. Godby, M. Schluter and L. J. Sham, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 10159 CrossRef; (b) C. S. Wang and B. M. Klein, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 24, 3417 CrossRef CAS; (c) M. S. Hybertsen and S. G. Louie, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 34, 5390 CrossRef CAS.
- S. N. Rashkeev, W. R. L. Lambrecht and B. Segall, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 3905 CrossRef CAS.
- (a) R. W. Godby, M. Schlueter and L. J. Sham, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 10159 CrossRef; (b) O. Gunnarsson and K. Schoenhammer, Phys. Rev. Lett., 1986, 56, 1968 CrossRef CAS PubMed.
- (a) C. Aversa and J. E. Sipe, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 52, 14636–14645 CrossRef CAS; (b) J. Lin, M. H. Lee, Z. P. Liu, C. Chen and C. J. Pickard, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 13380–13389 CrossRef CAS.
- (a) Q. Jing, X. Dong, Z. Yang, S. Pan, B. Zhang, X. Huang and M. Chen, J. Solid State Chem., 2014, 219, 138–142 CrossRef CAS; (b) F. Zhang, F. Zhang, J. Qun, S. Pan, Z. Yang and D. Jia, Phys. Chem. Chem. Phys., 2015, 17, 10489–10496 RSC.
- C. L. Christ and J. R. Clark, Phys. Chem. Miner., 1977, 2, 59–87 CrossRef CAS.
- I. D. Brown, Chem. Rev., 2009, 109, 6858–6919 CrossRef CAS PubMed.
- (a) C. Heyward, C. D. McMillen and J. W. Kolis, J. Chem. Crystallogr., 2013, 43, 96–102 CrossRef CAS; (b) E. L. Belokoneva, E. A. Ruchkina and O. V. Dimitrova, Russ. J. Inorg. Chem., 2001, 46, 20–27 Search PubMed.
- (a) C. Heyward, C. McMillen and J. Kolis, Inorg. Chem., 2012, 51, 3956–3962 CrossRef CAS PubMed; (b) Z. Y. Wu, P. Brandao and Z. Lin, Inorg. Chem., 2012, 51, 3088–3093 CrossRef CAS PubMed.
- J. P. Perdew and M. Levy, Phys. Rev. Lett., 1983, 51, 1884–1887 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Related methodologies of single crystal XRD, powder XRD, and IR spectrum measurements; crystallographic data in cif format, relevant crystallographic data, atomic coordinates and thermal parameters, and selected bond lengths and angles; microphotography, experimental and calculated powder XRD pattern, and IR spectrum of Pb6[B4O7(OH)2]3. See DOI: 10.1039/c6ra23193d |
| This journal is © The Royal Society of Chemistry 2016 |