Open Access Article
Open Access ArticleSupport effects on SILP hybrid catalysts prepared with carbon materials and the RhCOD complex†
M.
Rufete-Beneite
and
M. C.
Román-Martínez
*
Department of Inorganic Chemistry and Materials Institute, University of Alicante, Carretera de San Vicente s/n, E-03690 Alicante, Spain. E-mail: mcroman@ua.es
First published on 18th October 2016
Abstract
The Rh complex RhCOD has been immobilized on different carbon materials by means of the SILP methodology (Supported Ionic Liquid Phase) with the purpose of preparing hybrid catalysts (those combining the advantages of homogeneous and heterogeneous catalysis). The catalysts prepared in this work have been tested in the hydrogenation of cyclohexene showing good activity, and being all of them more active than an equivalent biphasic system. They are reusable without significant loss of activity in at least three runs. An important effect of the support properties, related to the pore texture and the surface chemistry, has been found. Likely, an appropriate combination of high supermicropore volume, high mesopore volume and a reach surface chemistry leads to the best results. The obtained results should stimulate analogous research on the challenging development of chiral SILP catalysts.
1. Introduction
The immobilization of a homogeneous catalyst on a solid support is a process by which the active species becomes heterogenized, and in this way the system combines the main advantages of homogeneous and heterogeneous catalysis. Those advantages are, respectively, high activity and selectivity, and easiness to recover and reuse the catalyst. Because of this combination of properties, heterogenized homogeneous catalysts are also known as hybrid catalysts.1–5Preparation of hybrid catalysts can be accomplished by means of strategies like the development of covalent bonds or the setting-up of electrostatic interactions between the metal complex and the support surface.2,6–8 Another approach is the SILP (Supported Ionic Liquid Phase) methodology by which the molecular homogeneous catalyst is dissolved in a certain amount of ionic liquid (IL) and the solution is supported on a solid material.9–12 Several applications of SILP systems have been reported recently.13 Ionic liquids are organic salts, in liquid state at room temperature, that have very good capabilities as solvents14 and interesting applications in catalysis,15–20 including biocatalysis.21 It must be mentioned that SILP catalysts are being considered by the industry as a good alternative to develop immobilized homogeneous catalysts.22
In SILP catalysts, the active species suffers a relatively low modification of its chemical and physical properties and thus its nature (chemical and conformational) can be preserved. This is an advantage compared to other methodologies and because of that the SILP method can be of particular interest in the immobilization of molecular chiral catalysts for which the steric configuration must be highly preserved.23 On the other hand, compared to a biphasic system, which could be also a way to immobilize and recover the homogeneous catalysts, the SILP samples offer a larger interphase for the catalytic process to take place. Besides, the support surface–IL interphase plays an important role in the catalyst activity and stability.
The SILP methodology, as all hybrid catalysts preparative strategies, must face the problems usually linked to the immobilization process: instability, often related with leaching, and the potential modification of the active phase which could lead to a loss of activity and/or selectivity. In general, the textural and chemical surface properties of the support have a high influence in the performance of supported catalysts. In the case of the SILP catalysts, the support plays a relevant role, primarily, in the interaction of the active phase IL solution with the solid surface, but also in the interfacial interaction between the mentioned supported solution and the reaction media. Among the several solids that can be used as catalysts supports,24 carbon materials are particularly suitable because they can be prepared with large surface area and appropriated pore size, and their surface chemistry can be tuned. Then, this kind of supports enables a good dispersion of the active species, proper diffusion of reactants and products and the regulation of the interaction of the active species with the support. Besides, they are stable in many reaction media and expensive supported noble metals can be recovered by support combustion. Some reviews on the use of carbon materials as catalyst supports, either for common heterogeneous catalysts25–28 or for metal complexes in general4,5 can be found in the literature. There are, as well, some works dealing with the use of carbon materials to prepare SILP catalysts.29–33
In the present case, SILP catalysts have been prepared using four different carbon materials (two spherical activated carbons, a powder activated carbon and a carbon black), the ionic liquid 1-butyl-3-methyl imidazolium hexafluorophosphate ([bmim][PF6]), and the Rh complex chloro(1,5-cyclooctadiene)rhodium(I) dimer, known as RhCOD. In order to investigate the catalytic properties of the prepared catalysts and to determine the effect of the support properties, the hybrid catalysts have been tested in the hydrogenation of cyclohexene in toluene, which has been selected as a test reaction. Thus, the general purpose of this work is to determine the feasibility of carbon based SILP systems by means of a test reaction, with the target of the further development of asymmetric SILP catalysts with the same supports. Also, a more specific purpose is to analyse which are the properties of the support that determine the catalytic activity and stability of the SILP hybrid catalysts.
2. Experimental
Carbon materials description and characterisation
The following commercial carbon materials have been used as support:GeA: spherical shaped activated carbon prepared from a phenolic resin by Gun-ei Chemical Industry (Japan). The spheres have an average diameter of 150 μm.
KA: spherical shaped activated carbon produced from petroleum pitch by Kureha Corporation (Japan). The spheres have an average diameter of 780 μm.
T: carbon black T-10157 from Columbian Chemical Company (USA).
SA: powder activated carbon SA-30 from MeadWestvaco (USA).
The spherical activated carbons KA and GeA have been selected because they are very easy to manipulate. The sphere size and the textural and chemical properties of these two samples are different (see below). Carbons T and SA are common activated carbons used as catalysts supports. They differ in their porous structure and surface chemistry (see below).
The textural properties have been studied by gas adsorption (N2 at 77 K and CO2 at 273 K) after degasification (vacuum, 423 K, 5 h), using an Autosorb 6-B apparatus. N2 adsorption data have been used to calculate: (i) the BET surface area, (ii) the total pore volume (Vtotal) from the amount of nitrogen adsorbed at P/P0 = 0.99, (iii) the micropore volume (Vμp) by applying the Dubinin–Radushkevich equation, and (iv) the mesopore volume (up to 7 nm, Vmeso) as the difference between the adsorbed volume at P/P0 = 0.90 and at P/P0 = 0.20.34,35 CO2 adsorption data have been used to calculate the volume of narrow micropores36,37 (diameter smaller than 0.7 nm, Vnμp) by applying the Dubinin–Radushkevich equation. The volume of supermicropores (Vsμp (0.7 nm < d < 2 nm)) is obtained from the difference between micropore volume (Vμp) and narrow micropore volume (Vnμp).34
The surface chemistry of the carbon materials has been assessed by Temperature Programmed Desorption (TPD) experiments (10 K min−1 in He, 100 cm3 min−1, up to 1273 K). The equipment used is a thermobalance TG-DTA (Mettler Toledo) coupled to a mass spectrometer (Thermostar GSD301T, Pfeiffer Vacuum).
Catalysts preparation and characterization
The catalysts were prepared with the ionic liquid 1-butyl-3-methyl imidazolium hexafluorophosphate ([bmim][PF6]) and the complex chloro(1,5-cyclooctadiene) rhodium(I) dimer (RhCOD) as follows: in a Schlenk apparatus, the appropriate amounts of ionic liquid (IL) and RhCOD to achieve 20 wt% IL and 1 wt% Rh loadings, and 2 cm3 anhydrous acetone, were added to 0.5 g of the outgassed support (vacuum, 423 K, 5 h). The mixture was kept under Ar atmosphere and stirring until the sample took on the original dry aspect of the support.Catalysts nomenclature is SUP20-RhCOD, where SUP accounts for the name of the support and 20 means 20 wt% IL ([bmim][PF6]) loading.
To analyse the role of the ionic liquid in the properties of the supported Rh complex, a catalyst was prepared without ionic liquid using support KA as follows: an acetone solution (2 cm3) with the necessary amount of RhCOD to achieve a 1 wt% Rh was added to the outgassed support and the mixture was kept in Ar atmosphere at room temperature with stirring to remove the solvent. This catalyst was named KA-RhCOD.
The amount of Rh in fresh and used catalysts was determined by ICP. The solution analysed by ICP was prepared as follows: about 30 mg of the catalyst were treated with a mixture H2SO4 (95%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 (69%) (2
HNO3 (69%) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), under reflux, until the solid was completely decomposed. Then, a 1
1), under reflux, until the solid was completely decomposed. Then, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 HNO3 (69%)
3 HNO3 (69%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl (37%) solution was added to the flask and it was heated until the volume was reduced to approximately 3 cm3. Finally, distilled water was added up to 10 cm3. Determinations on the catalysts prepared showed that the Rh loading is between 0.95 and 0.98 wt%, and thus it can be concluded that the catalysts have the desired amount of Rh.
HCl (37%) solution was added to the flask and it was heated until the volume was reduced to approximately 3 cm3. Finally, distilled water was added up to 10 cm3. Determinations on the catalysts prepared showed that the Rh loading is between 0.95 and 0.98 wt%, and thus it can be concluded that the catalysts have the desired amount of Rh.
Fresh and used catalysts were also analysed by X-ray photoelectron spectroscopy (XPS) using a VG-Microtech Multilab 3000 equipment, and by Transmission Electron Microscopy (TEM) using a JEM-2010 JEOL microscope.
Ionic liquid leaching was investigated by thermogravimetric analysis (TG) of the used catalysts in the following conditions: heating at 10 K min−1 up to 1073 K in N2 flow (100 cm3 min−1), using the equipment TA Instruments 2926; the weight loss registered corresponds to the amount of ionic liquid remaining on the catalysts after its use in a catalytic activity run.
Catalytic activity measurements and analysis
Catalysts were tested in the hydrogenation of cyclohexene in toluene in the following conditions: 30 mg catalyst, 5 vol% solution of cyclohexene in toluene (10 cm3) (S/C close to 1500), 10 bar H2, 333 K and magnetic stirring. The stainless steel reactor was heated by means of a polyethylene glycol bath. After a reaction time of 1.5 or 5 h, the catalysts were recovered by filtration in order to reuse them (under the same conditions) or to perform their characterisation.For a reference biphasic system, the catalyst was a solution of 1.5 mg of RhCOD (amount contained in 30 mg of the hybrid catalyst) in 0.01 cm3 of ionic liquid. A homogeneous phase test was also carried out dissolving 1.5 mg of RhCOD in the substrate solution.
A blank experiment with 10 cm3 of a 5 vol% solution of cyclohexene in toluene and 0.01 cm3 of ionic liquid was also performed (333 K, 10 bar H2, 5 h). In this case no cyclohexene conversion was observed.
Analysis was performed by gas chromatography using the equipment HP6890 Series II and the column HP-1 methylsiloxane 30 m × 250 μm × 0.25 μm. For the analysis, 0.150 cm3 of the problem solution were mixed with 0.250 cm3 acetone and 0.100 cm3 of a 7285 ppm solution of decane (internal standard) in acetone.
GC analysis parameters are included as ESI.†
3. Results and discussion
Textural properties of carbon materials
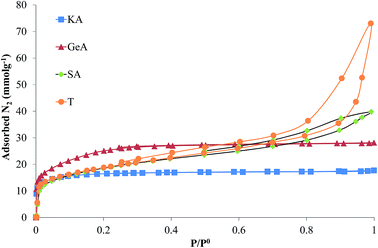
Fig. 1 shows the N2 adsorption isotherms (77 K) obtained for the four carbon materials used as support. These data reveal that they have widely different porosity developments and pore size distributions.The N2 adsorption isotherms of samples KA and GeA are type I according to the IUPAC classification, typical of microporous solids. The isotherms of samples SA and T are type I + IV, characteristic of solids with micro and mesoporosity.35
Table 1 shows a summary of the textural properties calculated from N2 adsorption (77 K) and CO2 adsorption (273 K) as indicated in the Experimental section.
| Sample | Textural properties | Oxygen surface chemistry | |||||||
|---|---|---|---|---|---|---|---|---|---|
| S BET (m2 g−1) | V total a (cm3 g−1) | V μp b (cm3 g−1) | V nμp c (cm3 g−1) | V sμp d (cm3 g−1) | V meso e (cm3 g−1) | CO2 (μmol g−1) | CO (μmol g−1) | O wt% | |
| a Total pore volume, N2 volume adsorbed at P/P0 = 0.99. b Micropore volume calculated by the Dubinin–Radushkevich equation applied to the N2 adsorption isotherm (77 K). c Narrow micropore (<0.7 nm) volume calculated by the Dubinin–Radushkevich equation applied to the CO2 adsorption isotherm (273 K). d Supermicropore (0.7 < d < 2 nm) volume calculated from the difference between micropore volume and narrow micropore volume. e Mesopore volume calculated by the difference between the volume of adsorbed N2 at P/P0 = 0.90 and P/P0 = 0.20. | |||||||||
| KA | 1291 | 0.67 | 0.55 | 0.44 | 0.11 | 0.05 | 291 | 167 | 1 |
| GeA | 1918 | 0.98 | 0.83 | 0.50 | 0.33 | 0.10 | 790 | 960 | 4 |
| SA | 1494 | 1.38 | 0.62 | 0.30 | 0.32 | 0.50 | 404 | 1720 | 4 |
| T | 1491 | 2.53 | 0.60 | 0.34 | 0.26 | 0.58 | 179 | 505 | 1 |
Although all supports have a large surface area, samples KA and GeA are essentially microporous while samples SA and T contain a significant amount of both micro and mesopores.
As previously published,38 and in agreement with what happens with other supports,39 the total pore volume determines the maximum amount of ionic liquid that these carbon materials can accept. Besides, it has been also reported38 that the loaded IL leads to some blocked porosity (not accessible to N2).
Oxygen surface chemistry
Table 1 also shows the amount of CO2 and CO evolved (in μmol g−1) from the different supports during TPD experiments, and the total amount of atomic oxygen (calculated from the amount of CO2 and CO evolved) expressed as wt%.Data of Table 1 show important differences between the samples. Supports GeA and SA can be pointed out as those with the richest surface chemistry, meaning this expression the largest amount of oxygen surface complexes.
The TPD profiles can be found as ESI (Fig. S1†).
Activity results
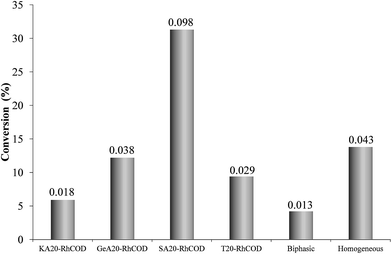
Fig. 2 shows values of cyclohexene conversion obtained with the hybrid SILP catalysts, and with the homogeneous and biphasic systems, at a reaction time of 1.5 h. Calculated TOF data (at t = 1.5 h and taking the active sites as moles of Rh from the Rh loading) have been also included.It can be observed that the homogeneous system is more active than the biphasic one, what suggests that the presence of ionic liquid around the complex hinders the access of reactants to the active centre. However, all hybrid catalysts are more active than the biphasic system, and a quite important effect of the support on the catalytic activity is observed. It is striking that the catalyst prepared with the activated carbon SA is even more active than the homogeneous system.
The activity values obtained in this study are similar or even higher than those reported for this reaction in previous works dealing with hybrid catalysts prepared with other metal complexes and supports. For example, under similar reactions conditions (toluene, 353 K, 11 bar H2), hybrid catalysts prepared by the adsorption of a Pd complex with tridecylamine ligands on carbon nanotubes, give TOF values between 0.0013 and 0.024 s−1 depending on the surface chemistry and the opening of the nanotubes.40 In similar conditions, catalysts prepared with a complex derived from the Wilkinson's catalyst and anchored by ionic exchange on different aluminosilicates, give TOF values between 0.028 and 0.036 s−1.41,42
Nevertheless, in order to give the right value to the obtained results, it is necessary to analyse the stability of the catalysts against leaching. This study is presented in the next paragraph.
Also, it is important to determine if the hybrid catalysts are reusable. Thus, the prepared catalysts were tested in three consecutive runs (of 1.5 and 5 h) to study their reusability. To reuse a catalyst, after a certain reaction time, it was removed from the reaction media by filtration and then used again in the same conditions (333 K, 10 bar H2). The obtained conversion data are presented in Table 2.
| Time (h) | Run | Conversion (%) | |||
|---|---|---|---|---|---|
| KA20-RhCOD | GeA20-RhCOD | SA20-RhCOD | T20-RhCOD | ||
| 1.5 | 1 | 5.9 | 12.2 | 31.3 | 9.4 |
| 2 | 12.5 | 8.1 | 32.6 | 12.2 | |
| 3 | 13.7 | 13.3 | 28 | 11.3 | |
| 5 | 1 | 52.9 | 92.8 | 100 | 100 |
| 2 | 68.9 | 86.4 | 100 | 100 | |
| 3 | 65.9 | 97.1 | 100 | 100 | |
It can be observed that all the catalysts keep the conversion in three consecutive runs meaning that they can be considered reusable. It is also worth to mention that in most cases an almost full conversion is reached in 5 h.
Comparison of data at 1.5 and 5 h reveals different kinetics for these catalysts. For example, samples GeA20-RhCOD and T20-RhCOD give a similar conversion at 1.5 h but the full conversion is reached earlier with the second one. Considering data of Table 2, the order in catalytic activity of these catalysts is as follows:
| KA20-RhCOD < GeA20-RhCOD < T20-RhCOD < SA20-RhCOD |
Regarding catalyst KA-RhCOD, prepared without ionic liquid, the obtained cyclohexene conversion at 1.5 h was 16.3% (versus the average 10.9% obtained with sample KA20-RhCOD in three consecutive runs, see Table 2). However, as it will be shown later, this catalyst is less stable against Rh leaching, meaning that, likely, the process takes place partially in homogenous phase.
Stability against leaching
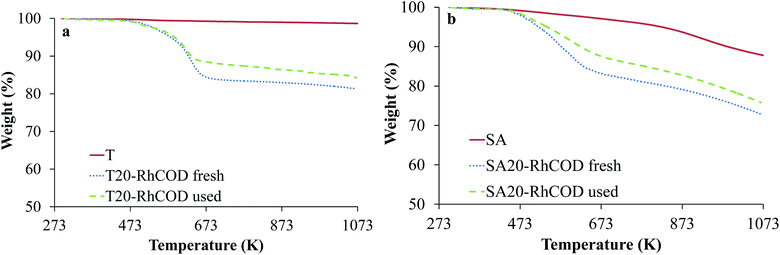
Fig. 3 shows as an example the TG profiles obtained for catalysts SA20-RhCOD and T20-RhCOD fresh and used (in a 5 h catalytic test), and for the original corresponding supports.
Table 3 collects the IL leaching data determined from the TG profiles.
These data are: the amount of IL leached in each case (in g of IL per gram of sample) and the difference between the IL loading in original and spent samples, meaning that the actual IL loading in each spent sample is 20 minus the given data.
It can be observed that the stability of these samples against IL leaching is strongly influenced by the support properties. To study this influence, the following has been considered: assuming that the cation and the anion are located as close as possible to each other, the size of one IL unit (cation + anion) has been estimated to be approximately 0.7 nm, so it can be expected that adsorption would be limited to happen in pores with diameter larger than 0.7 nm. It can be also assumed that the ionic liquid retained after reaction is strongly adsorbed on the support surface, likely due to its location in small pores. Thus, it is expected that the ionic liquid would be located in micropores larger than 0.7 nm (supermicropores (pores with diameter between 0.7 and 2 nm)) or in small mesopores.
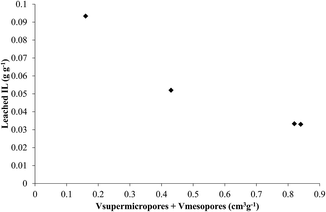
Taking all this into account, the amount of leached IL has been plotted versus the sum of supermicropore and mesopore volumes (values included in the figure) (data of Table 1) and the graphic is shown in Fig. 4.
According to these data, there is an acceptable inverse relationship between the amount of IL leached and the volume of supermicro- and mesopores of the supports, meaning that the supports with larger volumes of supermicro- and mesopores can retain more effectively the ionic liquid. This reveals an important effect of the support porosity in the catalyst stability.
It must be mentioned that a similar plot against micropore or mesopore volumes independently didn't give a correlation between leached IL and pore volume.
| Catalyst | Actual Rh leachinga | Theoretical Rh leachingb (μmol g−1) | |
|---|---|---|---|
| (%) | (μmol g−1) | ||
| a Determined by ICP. b Calculated from the ionic liquid leaching (Table 3), assuming that the solution of the complex in the ionic liquid is homogeneous. | |||
| KA20-RhCOD | 0.24 | 0.23 | 47.46 |
| KA-RhCOD | 16.00 | 15.55 | — |
| GeA20-RhCOD | 0.67 | 0.65 | 26.23 |
| SA20-RhCOD | 0.09 | 0.08 | 14.58 |
| T20-RhCOD | 0.17 | 0.16 | 16.30 |
In the four SILP catalysts, Rh leaching is very low (less than 0.7%), this amount of Rh leached would give less than 0.02 ppm in solution. Many studies report Rh leaching in these terms and conclude that it is negligible (usually below the detection level).10 On the contrary, the catalyst prepared without IL (sample KA-RhCOD) loses 16% of the original Rh loading. These data show that the ionic liquid helps to keep the complex supported, hindering leaching. The relatively high Rh leaching of catalyst KA-RhCOD means that in this case the hydrogenation reaction could have taken place partially in homogenous phase, being the reason of the higher activity of this sample commented before.
Assuming that the solution of the Rh complex in the supported ionic liquid is homogeneous and taking into account that its concentration is 0.03 M, a theoretical Rh leaching has been calculated from the IL leaching data. These values, also shown in Table 4, are much higher than those determined by analysis of the solution. The important difference between both sets of data indicates that the Rh complex is not homogeneously dissolved in the supported ionic liquid, and it can be assumed that the complex is mainly located close to the support surface with some interaction with it.
To check the effect of active phase leaching, blind tests have been carried out in some cases. After removal of the solid catalyst, fresh cyclohexene (0.5 cm3) was added to the solution remaining in the reactor, and then the system was submitted to the usual reaction conditions (333 K, 10 bar H2, 5 h). These tests have been done after using catalysts T20-RhCOD and SA20-RhCOD for 5 h. Cyclohexene conversion in the blind tests was lower than 5%, what confirms that complex leaching is very low in samples prepared with IL.
XPS and TEM characterization
As reported in some cases, the anchored complex in hybrid catalysts can be partially reduced under reaction conditions.43,44 To study if such transformation also occurs in this work, some of the used catalysts (1.5 h tests) have been analysed by XPS and TEM. Fresh catalysts have been also analysed in order to have an adequate reference. The binding energy found for Rh 3d5/2 (Table 5) corresponds to Rh(I) in both, fresh and used catalysts,48,49 meaning that the electronic state of Rh was not modified upon use (even after three consecutive reaction runs). However, it cannot be discarded that, as previously reported,44–47 the Rh complex could have suffered some modification related with the hydrogenation of the COD ligand.| Catalyst | B.E. (eV) | Catalyst | B.E. (eV) |
|---|---|---|---|
| a Fresh catalysts. b Used catalysts. c Catalysts used 3 consecutive runs. | |||
| KA20-RhCODfa | 309.6 | SA20-RhCODf | 309.6 |
| KA20-RhCODub | 309.7 | SA20-RhCODu | 309.6 |
| KA20-RhCODu3c | 309.7 | SA20-RhCODu3 | 309.7 |
| GeA20-RhCODfa | 309.5 | T20-RhCODf | 309.8 |
| GeA20-RhCODub | 309.7 | T20-RhCODu | 309.8 |
| GeA20-RhCODu3c | 309.5 | ||
Catalysts KA20-RhCOD and SA20-RhCOD (fresh and used) have been analysed by TEM and the obtained images are shown in Fig. 5.
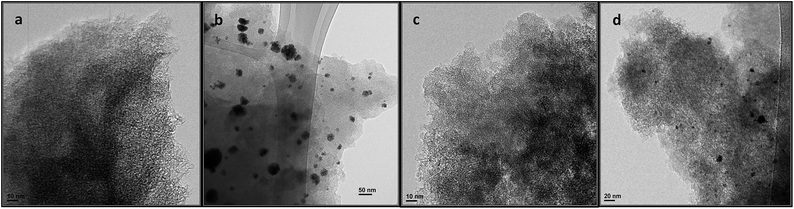 | ||
| Fig. 5 TEM images of catalyst KA20-RhCOD: (a) fresh and (b) used, and catalyst SA20-RhCOD: (c) fresh and (d) used (5 vol% cyclohexene in toluene, 333 K, 10 bar H2, 1.5 h). | ||
The TEM images of the fresh catalysts (Fig. 5(a) and (c)) do not show characteristics different from those of the original support, indicating that the fresh catalyst contains the complex well dissolved in the supported ionic liquid. However, in the used catalysts (Fig. 5(b) and (d)) some aggregates can be observed. The composition of these aggregates is not known but it is evident that they do not present the characteristic morphology of metallic particles. Similar structures have been observed in the case of a Pd complex dissolved in a ionic liquid and they have been attributed generically to Pd aggregates in which palladium is Pd+.30 Also in the case of Pd complexes, it has been suggested that solvent cages can be formed50 and this fact could also lead to these particular structures.
Comment on the support effects
The results presented and discussed above show that the SILP catalysts are more active than the analogous biphasic system and that they can be considered to be effective and reusable. In SILP catalysts the interface between the IL and the solvent used as reaction medium is higher than in the biphasic system and this is likely the ground of a higher activity.The different textural, chemical and morphological properties of the carbon materials used as supports led to marked differences in the activity of the catalysts prepared with them. The catalyst prepared with support SA (commercial activated carbon) is the most active (higher conversion in shorter time). Focusing the attention on the textural properties, the obtained results point to a positive effect of a developed porosity, with a relatively high volume of supermicro- and mesopores. The role of these two kinds of pores can be related to the location of the ionic liquid solution containing the active species, and also to the favoured diffusion of the substrate to the active sites.
Support T, in spite of having a volume of supermicropores and of mesopores similar to those of carbon SA, leads to a less active catalyst. The structure of the porosity (different in carbon blacks respect to activated carbons) may have an influence, but this is difficult to be evaluated. Another aspect to take into account is the different surface chemistry of supports SA and T, being the oxygen wt% 4 times higher in SA than in T (Table 1). Regarding this point, it should be considered that the polar/non polar interactions between the reaction media and the catalysts may also play a role (toluene has a certain polarity). The obtained data seem to indicate that the toluene solution interacts better with catalyst SA20-RhCOD than with catalyst T20-RhCOD.
In any case, a summary of the characteristics of support SA, the one that leads to the most active catalyst are: high supermicropore volume, high mesopore volume and a rich surface chemistry. In the other supports one or the three of these variables are low and thus, likely, it is a proper combination of them what leads to the best results.
4. Conclusions
SILP catalysts have been prepared with the complex RhCOD and the ionic liquid [bmim][PF6], using several carbon materials as support. They have been tested in the hydrogenation of cyclohexene and their properties have been compared with the corresponding homogeneous and biphasic systems. The hybrid catalysts show good activity, being all of them more active than an equivalent biphasic system. They are also more active than some previously reported hybrid catalysts. It is worth noting that an important effect of the support properties has been found, and the catalyst prepared with the activated carbon SA is the most active.Although some ionic liquid leaching takes place under reaction conditions Rh leaching is very low in all cases, generally lower than 1%. The ionic liquid helps to the stabilization of the catalyst, hindering Rh leaching.
In used catalysts, some aggregates, with structure and composition not determined, have been detected, but the reduction to Rh(0) nanoparticles can be ruled out.
The catalysts are reusable without significant loss of activity in at least three runs.
The effect of the support seems to be related with the pore texture and the surface chemistry. Likely, a proper combination of high supermicropore volume, high mesopore volume and a rich surface chemistry leads to the best results.
A practical conclusion is that by selecting the appropriate carbon material, the carbon based hybrid SILP catalysts can be active, stable against Rh leaching, and reusable. Besides, these results stimulate an analogous research to develop chiral SILP catalysts.
Acknowledgements
The authors thank the MINECO and FEDER for financial support through projects MAT2012-32832 and CTQ2015-66080-R, and GV for project PROMETEOII/2014/010. M. R. B. thanks the FPI scholarship grant associated to project MAT2009-07150.References
- Y. I. Yermakov, et al., Catalysis by Supported Metal Complexes, Stud. Surf. Sci. Cat., Elsevier, 1981, vol. 8 Search PubMed.
- A. Choplin, et al. , Coord. Chem. Rev., 1998, 178–180, 1679–1702 CrossRef CAS.
- A. P. Wight and M. E. Davis, Chem. Rev., 2002, 102, 3589–3614 CrossRef CAS PubMed.
- C. Freire and A. R. Silva, in Carbon Materials for Catalysis, ed. P. Serp and J. L. Figueiredo, John Wiley & Sons, Inc., New Jersey, 1st edn, 2009, ch. 8, pp. 267–308 Search PubMed.
- M. C. Román-Martínez and C. Salinas Martínez de Lecea, in New and Future Developments in Catalysis – Hybrid Materials, Composites, and Organocatalysts, ed. S. Suib, Elsevier, 1st edn, 2013, ch. 3, pp. 55–78 Search PubMed.
- M. Heitbaum, et al. , Angew. Chem., Int. Ed., 2006, 45, 4732–4762 CrossRef CAS PubMed.
- J. M. Fraile, et al. , Chem. Rev., 2009, 109, 360–417 CrossRef CAS PubMed.
- C. Copéret, et al. , Chem. Rev., 2016, 323–421 CrossRef PubMed.
- Y. Gu and G. Li, Adv. Synth. Catal., 2009, 351, 817–847 CrossRef CAS.
- C. Van Doorslaer, et al. , Dalton Trans., 2010, 39, 8377–8390 RSC.
- A. Riisager, et al. , Top. Catal., 2006, 40, 91–102 CrossRef CAS.
- T. Selvam, A. Machoke and W. Schwieger, Appl. Catal., A, 2012, 445–446, 92–101 CrossRef CAS.
- Supported Ionic Liquids: Fundamentals and Applications, ed. R. Fehrmann, A. Riisager and M. Haumann, Wiley-VCH, 2014 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- Q. Zhang, et al. , Green Chem., 2011, 13, 2619 RSC.
- H. Olivier-Bourbigou, et al. , Appl. Catal., A, 2010, 373, 1–56 CrossRef CAS.
- V. I. Pârvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615–2665 CrossRef PubMed.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS PubMed.
- J. Dupont and L. Kollár, Topics in Organometallic Chemistry, Springer, 2015, vol. 51 Search PubMed.
- T. Borkowski, et al. , J. Catal., 2014, 319, 87–94 CrossRef CAS.
- E. García-Verdugo, P. Lozano and L. V. Santiago, in Supported Ionic Liquids: Fundamentals and Applications, ed. R. Fehrmann, A. Riisager and M. Haumann, Wiley, 2014, ch. 17, pp. 351–368 Search PubMed.
- R. Franke and H. Hahn, Quarterly Sciences Newsletter, Evonik Industries, 2015, pp. 18–23 Search PubMed.
- I. Podolean, et al. , Catal. Today, 2013, 200, 63–73 CrossRef CAS.
- A. B. Stiles, Catalyst supports and supported catalysts: theoretical and applied concepts, Butterworth-Heinemann, 1987 Search PubMed.
- F. Rodríguez-Reinoso and A. Sepúlveda-Escribano, in Carbon Materials for Catalysis, ed. P. Serp and J. L. Figueiredo, John Wiley & Sons, Inc., New Jersey, 1st edn, 2009, ch. 4, pp. 131–156 Search PubMed.
- E. Auer, et al. , Appl. Catal., A, 1998, 173, 259–271 CrossRef CAS.
- V. Arunajatesan, et al., in Carbon Materials for Catalysis, ed. P. Serp and J. L. Figueiredo, John Wiley & Sons, Inc., New Jersey, 1st edn, 2009, ch. 15, pp. 535–572 Search PubMed.
- H. Jüntgen, Fuel, 1986, 65, 1436–1446 CrossRef.
- J. P. Mikkola, et al. , Ind. Eng. Chem. Res., 2007, 46, 3932–3940 CrossRef CAS.
- J. P. Mikkola, et al. , Appl. Catal., A, 2007, 328, 68–76 CrossRef CAS.
- E. J. García-Suárez, et al. , Microporous Mesoporous Mater., 2011, 144, 205–208 CrossRef.
- J. J. Y. Shin, et al. , Chem.–Asian J., 2011, 6, 2016–2021 CrossRef CAS PubMed.
- Y. S. Chun, et al. , Chem. Commun., 2008, 942–944 RSC.
- F. Rodríguez-Reinoso and A. Linares-Solano in Chemistry and Physics of Carbon, ed. P. Thorwer, Marcel Dekker, Inc., New York, 1989, vol. 21, pp. 1–146 Search PubMed.
- F. Rouquerol, J. Rouquerol and K. Sing, Adsorption by Powders and Porous Solids. Principles, Methodology and Applications, Academic Press, London, 1999 Search PubMed.
- D. Cazorla-Amorós, et al. , Langmuir, 1996, 12, 2820–2824 CrossRef.
- D. Lozano-Castelló, et al. , Carbon, 2004, 42, 1231–1236 Search PubMed.
- M. Rufete-Beneite, et al. , Carbon, 2014, 77, 947–957 CrossRef CAS.
- J. Lemus, et al. , Adsorption, 2011, 17, 561–571 CrossRef CAS.
- I. Such-Basáñez, et al. , Microporous Mesoporous Mater., 2016, 225, 378–384 CrossRef.
- I. Such-Basáñez, et al. , Curr. Catal., 2012, 1, 100–160 CrossRef.
- I. Such-Basáñez, PhD Thesis, University of Alicante, 2015.
- C. C. Gheorghiu, et al., in Studies in Surface Science and Catalysis, ed. E. M. Gaigneaux, et al., Elsevier, 2010, vol. 175, pp. 647–651 Search PubMed.
- C. C. Gheorghiu, et al. , ChemCatChem, 2013, 5, 1587–1597 CrossRef CAS.
- L. J. Lemus-Yegres, et al. , Appl. Catal., A, 2007, 331, 26–33 CrossRef CAS.
- M. A. D. N. Perera and R. J. Angelici, J. Mol. Catal. A: Chem., 1999, 149, 99–111 CrossRef CAS.
- A. Börner and D. Heller, Tetrahedron Lett., 2001, 42, 223–225 CrossRef.
- http://www.lasurface.com .
- http://srdata.nist.gov/xps/ .
- C. Sievers, et al. , J. Mol. Catal. A: Chem., 2008, 279, 187–199 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra22213g |
| This journal is © The Royal Society of Chemistry 2016 |