DOI:
10.1039/C6RA21914D
(Paper)
RSC Adv., 2016,
6, 91401-91408
Accessing highly linear polyethylenes by 2-(1-aryliminoethyl)-7-arylimino-6,6-dimethylcyclopenta[b]pyridylchromium(III) chlorides†
Received
1st September 2016
, Accepted 18th September 2016
First published on 19th September 2016
Abstract
A series of 2-(1-aryliminoethyl)-7-arylimino-6,6-dimethylcyclopenta[b]pyridylchromium(III) chlorides (aryl = 2,6-Me2Ph (Cr1), 2,6-Et2Ph (Cr2), 2,6-i-Pr2Ph (Cr3), 2,4,6-Me3Ph (Cr4), 2,6-Et2-4-MePh (Cr5)) was synthesized and characterized using FT-IR and elemental analysis, and the molecular structure of complex Cr1 was confirmed by using single-crystal X-ray diffraction to present a distorted octahedral geometry around the chromium center. Upon activation with modified methylaluminoxane (MMAO) or ethylaluminium sesquichloride (EASC), all chromium pre-catalysts exhibit good activities towards ethylene polymerization for the highly linear polyethylene.
Introduction
Among transition metal catalysts in polyolefins,1 chromium-based catalysts play a significant role1b,2 especially for the Phillips catalyst3 and Union Carbide catalyst4 producing one third of the commercial high-density polyethylenes (HDPE).5 To understand the active species and mechanism of olefin polymerization, various chromium catalysts have been extensively explored6 producing micro-structurally controlled polyolefins in homogeneous systems.7 In addition to cyclopentadienyl-based chromium precatalysts,8 Cp-free chromium complexes have drawn more attention because of easily available ligands with fine tuning of the stereo- and electronic properties.9 Various chromium pre-catalysts have been classified complexes with bidentate ligands such as N^O,10 N^N,11 and P^P,12 and tridentate analogs with active sites better stabilized13 such as N^N^N,14 N^N^O,9f,15 P^P^P,16 P^N^P,12a,17 S^N^S,17b,18 C^N^C19 and N^S^N.20 Interestingly the characteristic polyethylenes possessing high linearity or slight short branches were commonly achieved by the homogeneous catalysis on the base of bis(imino)pyridyl chromium precatalysts,21 highly linear polyethylenes without any branches are still under demanding. In addition, the modifications of the ligand backbone have been extensively investigated, and the cycloalkyl-fused pyridine derivatives including 2-(1-(arylimino)ethyl)-8-arylimino-5,6,7-trihydro quinoline,22 1,8-diimino-2,3,4,5,6,7-hexahydroacridines,23 and 2-(1-aryliminoethyl)-9-aryliminocyclohepta[b]pyridine24 have been developed for their iron and cobalt complexes as highly active pre-catalysts in ethylene polymerization. Recently, more rigidly geometric 2-(1-(arylimino)ethyl)-7-arylimino-6,6-dimethylcyclopenta[b]pyridine derivatives were synthesized to develop their corresponding cobalt complexes in ethylene polymerization.25 Subsequently the series of 2-(1-(arylimino)ethyl)-7-arylimino-6,6-dimethylcyclopenta[b]pyridylchromium(III) trichlorides is prepared and explored their catalytic behaviors toward ethylene polymerization. In the presence of MMAO or EASC, all chromium pre-catalysts exhibit good activities toward ethylene polymerization producing unique polyethylenes with highly linear feature. Herein the synthesis and characterization of the title chromium complexes are reported along with their ethylene polymerization.
Results and discussion
Synthesis and characterization
The 2-(1-(arylimino)ethyl)-7-arylimino-6,6-dimethylcyclopenta[b]pyridine derivatives (L1–L5)25 individually reacted with CrCl3(THF)3 (ref. 26) in acetone to form the corresponding complexes in the good yields between 68% and 76%. Compared with the organic compounds (L1–L5), the FT-IR spectra of these chromium complexes indicated the clear shift of ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) stretching vibrations into a lower field, illustrating the effective coordination between the imino-nitrogen and chromium atom. Moreover, their elemental analyses are consistent with Cr1–Cr5. In addition, the molecular structure of the complexes Cr1 was confirmed by single-crystal X-ray diffraction studies.
N) stretching vibrations into a lower field, illustrating the effective coordination between the imino-nitrogen and chromium atom. Moreover, their elemental analyses are consistent with Cr1–Cr5. In addition, the molecular structure of the complexes Cr1 was confirmed by single-crystal X-ray diffraction studies.
X-ray crystallographic studies
Single crystal of the chromium complex Cr1 suitable for X-ray diffraction analysis was obtained by slow diffusion of heptane into the dichloromethane solution at room temperature. The molecular structure is shown in Fig. 1, and their selected bond lengths and angles are tabulated in Table 1.
 |
| | Fig. 1 ORTEP drawing of complex Cr1. Thermal ellipsoids are shown at the 30% probability level, and H atoms and CH2Cl2 are omitted for clarity. | |
Table 1 Selected bond lengths (Å) and angles (°) for Cr1
| Cr1 |
| Bond lengths (Å) |
| Cr(1)–N(1) |
2.150(6) |
Cr(1)–Cl(2) |
2.347(2) |
| Cr(1)–N(2) |
1.985(6) |
Cr(1)–Cl(3) |
2.299(2) |
| Cr(1)–N(3) |
2.299(6) |
N(1)–C(2) |
1.320(9) |
| Cr(1)–Cl(1) |
2.297(2) |
N(3)–C(8) |
1.311(9) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Bond angles (°) |
| N(1)–Cr(1)–N(3) |
153.10(2) |
N(2)–Cr(1)–Cl(2) |
82.12(17) |
| N(1)–Cr(1)–N(2) |
75.90(2) |
N(2)–Cr(1)–Cl(3) |
175.26(18) |
| N(2)–Cr(1)–N(3) |
77.40(2) |
N(3)–Cr(1)–Cl(1) |
88.37(17) |
| N(1)–Cr(1)–Cl(1) |
89.00(17) |
N(3)–Cr(1)–Cl(2) |
89.25(17) |
| N(1)–Cr(1)–Cl(2) |
90.05(17) |
N(3)–Cr(1)–Cl(3) |
102.85(15) |
| N(1)–Cr(1)–Cl(3) |
103.99(17) |
C(2)–N(1)–Cr(1) |
115.50(5) |
| N(2)–Cr(1)–Cl(1) |
90.70(17) |
C(8)–N(3)–Cr(1) |
107.90(5) |
In the molecular structure of complex Cr1 (Fig. 1), X-ray analysis revealed a slightly distorted octahedron geometry around the chromium center in a meridional manner with the plane containing the three coordinated nitrogen atoms perpendicular to the plane containing the three chlorides. The N1, N3, Cl1 and Cl2 atoms could be described as forming the equatorial plane and the two axial bonds nearly formed a straight line through the metal centre (N(2)–Cr(1)–Cl(3), 175.26°). Similar structural features have been observed elsewhere for chromium complexes.14d,14e,27 Moreover, the two aryl–Nimino planes are twisted to the N^N^N coordination plane with dihedral angles of 72.11° and 79.03°, respectively. The bond lengths of Cr–Npyridyl are shorter than those of the Cr–Nimino bonds (Table 1), indicating the stronger bond of Cr–Npyridyl. The differences in bond length between two Cr–Nimino bonds are mainly attributed to the unsymmetric framework,28 which is consistent with the previous observation on the Co–Nimino bonds in the cobalt analogues;25 in addition, the Cr–Npyridyl bonds are shorter than the Co–Npyridyl bonds,25 exhibiting more robust coordination within the chromium complexes.
Ethylene polymerization
Complex Cr4 was explored with various alkylaluminium reagents such as MAO, MMAO, EASC and Et2AlCl at 30 °C under 10 atm of ethylene in order to find the most suitable co-catalysts and the results were exhibited in Table 2; all co-catalysts in general promoted good activities. The obtained polyethylenes were measured by gel permeation chromatography (GPC) and differential scanning calorimetry (DSC), indicating molecular weights in the range of 105 g mol−1 and the Tm values fall in a similar range (135.3–136.8 °C). The co-catalysts, being classified into aluminoxane and chloroaluminum type; for which the highest activities were attained, MMAO (entry 2, Table 2) and EASC (entry 4, Table 2), were selected for further detailed exploration.
Table 2 Selection of the suitable co-catalysts based on Cr4a
| Entry |
Co-cat. |
Al/Cr |
PE/g |
Activityb |
Mwc |
Mw/Mnc |
Tmd/°C |
| Reaction conditions: 5 μmol of Cr4; 30 °C; 30 min; 10 atm of ethylene; 100 mL total volume of toluene. 105 g(PE) mol−1(Cr) h−1. Determined by GPC, and Mw: 105 g mol−1. Determined by DSC. |
| 1 |
MAO |
2000 |
0.33 |
1.32 |
2.35 |
18.3 |
135.4 |
| 2 |
MMAO |
2000 |
1.11 |
4.44 |
5.12 |
15.7 |
136.8 |
| 3 |
Et2AlCl |
500 |
0.27 |
1.08 |
5.96 |
22.7 |
135.8 |
| 4 |
EASC |
500 |
0.61 |
2.44 |
3.36 |
10.4 |
135.3 |
Ethylene polymerization by Cr1–Cr5/MMAO
Regarding the catalytic system with MMAO, the complex Cr4 was chosen for optimizing the polymerization conditions, such as the reaction temperatures, the ratios of co-catalyst as well as reaction times, to evaluate how catalytic performances and polymer property can be influenced, and the obtained results are tabulated in Table 3. Firstly, the catalytic system was studied by varying the temperature from 20 °C to 60 °C (entries 1–5, Table 3) at an Al/Cr ratio of 2000 for 30 min, with the optimum temperature observed as 30 °C (entry 2, Table 3). Raising the temperature further resulted in a sharp decrease in activities, this being attributed to both the instability of the active species7,13,29 and the lower solubility of ethylene in toluene at higher temperature.30 Moreover, lower molecular weights and Tm values of the obtained polyethylenes were observed at higher reaction temperatures, being interpreted as fast chain termination at higher temperatures. Their GPC curves are shown in Fig. 2.
Table 3 Polymerization of ethylene in the presence of MMAOa
| Entry |
Pre-cat. |
T/°C |
Al/Cr |
t/min |
PE/g |
Activityb |
Mwc |
Mw/Mnc |
Tmd/°C |
| Reaction conditions: 5 μmol of Cr; 30 °C; 30 min; 10 atm of ethylene; 100 mL total volume of toluene. 105 g(PE) mol−1(Cr) h−1. Determined by GPC, and Mw: 105 g mol−1. Determined by DSC. Conditions: 5 atm of ethylene. |
| 1 |
Cr4 |
20 |
2000 |
30 |
0.83 |
3.32 |
5.16 |
16.8 |
137.1 |
| 2 |
Cr4 |
30 |
2000 |
30 |
1.11 |
4.44 |
5.12 |
15.7 |
136.8 |
| 3 |
Cr4 |
40 |
2000 |
30 |
0.58 |
2.32 |
3.91 |
16.4 |
136.6 |
| 4 |
Cr4 |
50 |
2000 |
30 |
0.44 |
1.76 |
2.81 |
11.5 |
136.1 |
| 5 |
Cr4 |
60 |
2000 |
30 |
0.17 |
0.68 |
2.16 |
24.1 |
135.1 |
| 6 |
Cr4 |
30 |
1500 |
30 |
0.51 |
2.04 |
4.74 |
15.4 |
135.8 |
| 7 |
Cr4 |
30 |
1750 |
30 |
0.59 |
2.36 |
5.99 |
14.4 |
135.4 |
| 8 |
Cr4 |
30 |
2250 |
30 |
0.62 |
2.48 |
5.06 |
18.7 |
136.3 |
| 9 |
Cr4 |
30 |
2500 |
30 |
0.48 |
1.92 |
4.98 |
19.7 |
134.9 |
| 10 |
Cr4 |
30 |
2000 |
5 |
0.12 |
2.88 |
3.37 |
17.6 |
134.4 |
| 11 |
Cr4 |
30 |
2000 |
10 |
0.53 |
6.36 |
3.70 |
10.5 |
136.7 |
| 12 |
Cr4 |
30 |
2000 |
15 |
0.59 |
4.72 |
4.13 |
9.9 |
136.5 |
| 13 |
Cr4 |
30 |
2000 |
45 |
1.26 |
3.36 |
5.43 |
14.8 |
135.1 |
| 14 |
Cr4 |
30 |
2000 |
60 |
1.42 |
2.84 |
6.48 |
17.7 |
136.8 |
| 15 |
Cr1 |
30 |
2000 |
30 |
0.30 |
1.20 |
1.60 |
15.5 |
127.5 |
| 16 |
Cr2 |
30 |
2000 |
30 |
0.32 |
1.28 |
1.84 |
16.2 |
129.8 |
| 17 |
Cr3 |
30 |
2000 |
30 |
0.39 |
1.56 |
5.75 |
17.2 |
135.5 |
| 18 |
Cr5 |
30 |
2000 |
30 |
0.48 |
1.92 |
2.65 |
18.8 |
134.4 |
| 19e |
Cr4 |
30 |
2000 |
30 |
0.52 |
2.08 |
3.46 |
9.9 |
136.1 |
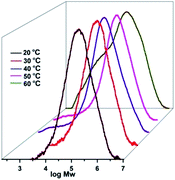 |
| | Fig. 2 GPC curves for the polyethylenes by the Cr4/MMAO system at different temperatures (entries 1–5 in Table 3). | |
With the reaction temperature fixed at 30 °C, the molar ratio of Al/Cr was investigated between 1500 to 2500 (entries 2 and 6–9, Table 3), the optimum performance reached 4.44 × 105 g(PE) mol−1(Cr) h−1 at the Al/Cr ratio of 2000 (entry 2, Table 3). Moreover, the highest molecular weight can be observed at the Al/Cr ratio of 1750 (entry 7, Table 3), on further increasing the Al/Cr molar ratios to 2500 (entries 2 and 8–9, Table 3), the molecular weights were decreased, and their GPC curves are illustrated in Fig. 3.
 |
| | Fig. 3 GPC curves for the polyethylenes by the Cr4/MMAO system with various Al/Cr ratios (entries 2 and 6–9 in Table 3). | |
To understand the lifetime of the active species, the catalytic system was terminated at different times such as 5, 10, 15, 30, 45 and 60 min (entries 2 and 10–14, Table 3) with the Al/Cr ratio fixed at 2000 and the temperature at 30 °C; the highest activity was observed within 10 min (entry 11, Table 3), probably reflecting that the active species were quickly formed with adding MMAO and the active species were gradually deactivated as the catalytic reaction time was prolonged.31
On the basis of the above observed activities as the highest, the optimum conditions were identified as the Al/Cr ratio of 2000 at 30 °C and a run time of 30 min. Using the optimized reaction conditions, the other pre-catalysts were investigated for their behaviors toward ethylene polymerization (entries 15–18, Table 3). Their activities showed the order Cr4 [2,4,6-tri(Me)] > Cr5 [2,6-di(Et)-4-Me] > Cr3 [2,6-di(i-Pr)] > Cr2 [2,6-di(Et)] > Cr1 [2,6-di(Me)]. The complexes Cr4 and Cr5 showed better activities than the corresponding analogous complexes Cr1 and Cr2, this can be ascribed to better solubility due to the presence of an additional para-methyl of the phenyl.32 In addition, the chromium pre-catalysts with bulky ortho-substituent showed higher catalytic activity, such a phenomenon probably illustrates the protection afforded to the active site by the sterically bulky substituent.27,33
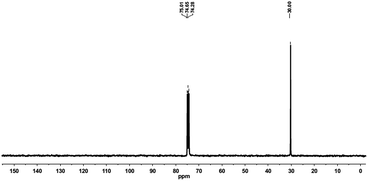
With regard to the properties of the polyethylenes obtained using Cr4, the GPC and DSC measurements indicated that the polyethylenes in general displayed high molecular weights, and the melting points of the resultant polyethylenes were generally higher than 127 °C, indicative of the feature of highly linear polyethylene. To verify the linearity, the polyethylene produced by Cr4/MMAO under the optimum conditions (entry 2 in Table 3) was characterized by 13C NMR spectrum (Fig. 4), and showed a single peak without observable branching components or visible vinylic end groups, confirming a highly linear polyethylene.34
 |
| | Fig. 4 13C NMR spectrum of the PE obtained with Cr4/MMAO (entry 2 in Table 3). | |
Ethylene polymerization by Cr1–Cr5/EASC
Using EASC as co-catalyst, the pre-catalyst Cr4 was again explored for the optimum polymerization parameters through varying the reaction temperatures, Al/Cr molar ratios and reaction times. The results are tabulated in Table 4, which showed a slightly lower activities and molecular weights as for those observed with the MMAO as the co-catalyst, whilst the resultant polyethylenes exhibited bimodal character in most cases.
Table 4 Polymerization of ethylene in the presence of EASCa
| Entry |
Pre-cat. |
T/°C |
Al/Cr |
t/min |
PE/g |
Activityb |
Mwc |
Mw/Mnc |
Tmd/°C |
| Reaction conditions: 5 μmol of Cr; 30 °C; 30 min; 10 atm of ethylene; 100 mL total volume of toluene. 105 g(PE) mol−1(Cr) h−1. Determined by GPC, and Mw: 105 g mol−1. Determined by DSC. Conditions: 5 atm of ethylene. |
| 1 |
Cr4 |
20 |
500 |
30 |
0.56 |
2.24 |
4.24 |
8.5 |
137.5 |
| 2 |
Cr4 |
30 |
500 |
30 |
0.61 |
2.44 |
3.36 |
10.4 |
135.8 |
| 3 |
Cr4 |
40 |
500 |
30 |
0.46 |
1.84 |
2.53 |
17.7 |
132.8 |
| 4 |
Cr4 |
50 |
500 |
30 |
0.32 |
1.28 |
0.95 |
21.9 |
132.2 |
| 5 |
Cr4 |
60 |
500 |
30 |
0.25 |
1.00 |
0.08 |
3.6 |
131.9 |
| 6 |
Cr4 |
30 |
400 |
30 |
0.44 |
1.76 |
2.42 |
9.7 |
134.6 |
| 7 |
Cr4 |
30 |
600 |
30 |
0.73 |
2.92 |
3.61 |
9.3 |
134.9 |
| 8 |
Cr4 |
30 |
700 |
30 |
0.81 |
3.24 |
3.65 |
8.1 |
135.1 |
| 9 |
Cr4 |
30 |
800 |
30 |
0.69 |
2.76 |
4.27 |
12.9 |
134.9 |
| 10 |
Cr4 |
30 |
900 |
30 |
0.56 |
2.24 |
3.07 |
9.5 |
135.2 |
| 11 |
Cr4 |
30 |
700 |
5 |
0.10 |
2.40 |
1.23 |
9.2 |
133.1 |
| 12 |
Cr4 |
30 |
700 |
10 |
0.27 |
3.24 |
1.69 |
7.9 |
133.9 |
| 13 |
Cr4 |
30 |
700 |
15 |
0.43 |
3.44 |
2.23 |
9.4 |
135.5 |
| 14 |
Cr4 |
30 |
700 |
45 |
1.01 |
2.69 |
4.16 |
7.4 |
136.1 |
| 15 |
Cr4 |
30 |
700 |
60 |
1.16 |
2.32 |
4.77 |
8.0 |
136.4 |
| 16 |
Cr1 |
30 |
700 |
30 |
0.41 |
1.64 |
1.41 |
7.9 |
133.8 |
| 17 |
Cr2 |
30 |
700 |
30 |
0.57 |
2.28 |
1.82 |
5.9 |
135.1 |
| 18 |
Cr3 |
30 |
700 |
30 |
1.41 |
5.64 |
2.82 |
6.2 |
135.8 |
| 19 |
Cr5 |
30 |
700 |
30 |
0.75 |
3.00 |
1.98 |
6.8 |
135.6 |
| 20e |
Cr4 |
30 |
700 |
30 |
0.25 |
1.00 |
2.34 |
14.2 |
134.8 |
With a molar ratio of Al/Cr fixed at 500 (entries 1–5, Table 4) under 10 atm of ethylene, the reaction temperature was explored and the optimum was found to be 30 °C with the activity of 2.44 × 105 g(PE) mol−1(Cr) h−1. As the Cr4/MMAO system, raising the temperature further resulted in a dramatically decrease in activities, and lower molecular weights and Tm values of the obtained polyethylenes were observed at higher reaction temperatures. The GPC curves of the resultant polyethylenes in Fig. 5 showed that the polyethylene obtained at a higher temperature (60 °C) possessed a unimodal character, while polyethylenes obtained at lower temperatures tended to be bimodal.
 |
| | Fig. 5 GPC curves for the polyethylenes by the Cr4/EASC system at different temperatures (entries 1–5 in Table 4). | |
With regard to the molar ratios of Al/Cr, higher activities were gradually observed along with increasing the Al/Cr molar ratio from 400 to 700 (entries 2 and 6–8, Table 4), and the system at the Al/Cr ratio of 700 showed the best activity up to 3.24 × 105 g(PE) mol−1(Cr) h−1; however, on further increasing the Al/Cr molar ratio to 900 (entries 9–10, Table 4), the catalytic activities were slightly decreased. Bimodal polyethylenes were the characteristic feature of the polyethylenes observed at the higher molar ratios of Al/Cr (Fig. 6), indicating multiple active species formed with more aluminium co-catalyst.
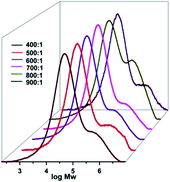 |
| | Fig. 6 GPC curves for the polyethylenes by the Cr4/EASC system with various Al/Cr ratios (entries 2 and 6–10 in Table 4). | |
Concerning the lifetime of active species, the catalytic system was terminated at different times (entries 8 and 11–15, Table 4); the highest activity was observed within 15 min (entry 13, Table 4). Moreover, the resultant polyethylenes exhibited higher molecular weights and Tm values as the catalytic reaction time was prolonged.
All the other chromium pre-catalysts were investigated in the presence of EASC, and were found to perform with good activities in ethylene polymerization under the optimum conditions of Al/Cr molar ratio 700 at 30 °C (entries 8 and 16–19, Table 4). Their activities showed the order Cr4 [2,4,6-tri(Me)] > Cr5 [2,6-di(Et)-4-Me] > Cr3 [2,6-di(i-Pr)] > Cr2 [2,6-di(Et)] > Cr1 [2,6-di(Me)], which was consistent with the observation of the catalytic system with MMAO of complexes Cr1–Cr5. In addition, the polyethylene produced by Cr4/EASC under the optimum conditions (entries 8, Table 4) was examined by 13C NMR spectroscopy (Fig. 7), and indicated the feature of highly linear polyethylene.
 |
| | Fig. 7 13C NMR spectrum of the PE obtained with Cr4/EASC (entry 8 in Table 4). | |
Conclusion
In conclusion, a series of 2-(1-aryliminoethyl)-7-arylimino-6,6-dimethylcyclopenta[b]pyridylchromium chlorides complexes was synthesized and characterized using FT-IR and elemental analysis, and the molecular structure of complex Cr1 was confirmed using signal-crystal X-ray diffraction. On activation with MMAO or EASC, all title chromium complexes showed good activities in ethylene polymerization with an activity of up to 4.44 × 105 g(PE) mol−1(Cr) h−1, and the obtained polyethylene possessed high linearity.
Experimental section
General procedures
All works involving air- and/or moisture-sensitive compounds were performed in an atmosphere of nitrogen using standard Schlenk techniques. Before being used, toluene was refluxed over sodium-benzophenone and distilled under nitrogen. Methylaluminoxane (MAO, 1.46 M in toluene) and modified methylaluminoxane (MMAO, 1.93 M in n-heptane) were purchased from Akzo Nobel Corp. Diethylaluminium chloride (Et2AlCl, 1.17 M in toluene) and ethylaluminium sesquichloride (EASC, 0.87 M in n-hexane) was purchased from Acros Chemicals. High-purity ethylene was purchased from Beijing Yanshan Petrochemical Co. and used as received. Other reagents were purchased from Aldrich, Acros, or local suppliers. NMR spectra were recorded on a Bruker DMX 400 MHz instrument at ambient temperature using TMS as an internal standard. IR spectra were recorded on a Perkin-Elmer System 2000 FT-IR spectrometer. Elemental analysis was carried out using a Flash EA 1112 microanalyzer. Molecular weights and molecular weight distribution (MWD) of polyethylenes were determined by the Agilent PL-GPC220 GPC/SEC High Temperature System. The columns are three 300 × 7.5 mm PLgel 10 μm MIXED-B LS columns connected in series. The testing was undertaken at 150 °C with a flow rate of 1.0 mL min−1. The eluent was 1,2,4-trichlorobenzene(TCB). Data collection and handling were carried out using Cirrus GPC Software and Multi Detector Software. Data were collected at 1 point per second. The calibrants for constructing conventional calibration were from the Polystyrene Calibration Kit S-M-10 from PL Company. The true average molecular weights of PE are transferred by inputting the M–H constants of PE; K of 0.727 and α of 40.6 were provided by PL Company. Samples were dissolved at a concentration of 0.5–2.5 mg mL−1, depending on the molecular weights. The melting points of polyethylenes were measured from the second scanning run on a PerkinElmer TA-Q2000 differential scanning calorimetry (DSC) analyzer under a nitrogen atmosphere. In the procedure, a sample of about 5.0 mg was heated to 160 °C at a rate of 20 °C min−1 and kept for 2 min at 160 °C to remove the thermal history and then cooled at a rate of 20 °C min−1 to −40 °C. 13C NMR spectra of the polyethylenes were recorded on a Bruker DMX 300 MHz instrument at 100 °C in deuterated 1,1,2,2-tetrachloroethane with TMS as an internal standard.
According to the synthetic procedures in the literature,25 the organic compounds (L1–L5) were prepared in good yields (Scheme 1).
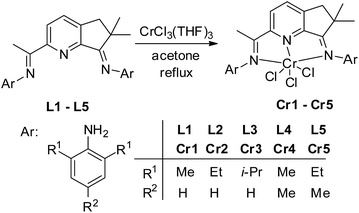 |
| | Scheme 1 Synthetic procedure for L1–L5 and Cr1–Cr5. | |
Synthesis of 2-(1-(2,6-dimethylphenylimino)ethyl)-7-(2,6-dimethylphenylimino)-6,6-dim ethylcyclopenta[b]pyridylchromium chloride (Cr1). CrCl3(THF)3 (0.27 mmol) added to the solution of the 2-(1-(2,6-dimethylphenylimino)ethyl)-7-(2,6-dimethylphenylimino)-6,6-di methylcyclopenta[b]pyridine (L1) (0.3 mmol) in 10 mL acetone. The mixture was stirred for 6 h at reflux temperature, and then excess diethyl ether was poured into the mixture to precipitate the complex. The precipitant was collected by filtration, washed with diethyl ether and dried under vacuum to give the product as green powder in 68.0% yield. FT-IR (KBr, cm−1): 3069.4 (w), 2961.2 (w), 2915.8 (m), 1630.1 (m), 1603.7 (m), 1585.1 (m), 1558.3 (w), 1463.1 (s), 1423.2 (m), 1359.2 (m), 1236.0 (w), 1221.4 (s), 1191.6 (s), 1092.4 (m), 1029.8 (w), 901.1 (m), 840.7 (m), 789.6 (s), 771.2 (s), 657.0 (m). Anal. Calcd for C28H31N3CrCl3: C, 59.22; H, 5.50; N, 7.40. Found: C, 58.79; H, 5.30; N, 7.14.
Synthesis of 2-(1-(2,6-diethylphenylimino)ethyl)-7-(2,6-diethylphenylimino)-6,6-dimethylcyclopenta[b]pyridylchromium chloride (Cr2). In a manner similar to that described for Cr1, Cr2 was isolated as green powder 75.5% yield. FT-IR (KBr, cm−1): 2962.2 (m), 2921.9 (w), 2875.7 (m), 1629.5 (m), 1600.6 (m), 1570.4 (w), 1556.5 (m), 1455.5 (s), 1371.4 (m), 1340.9 (w), 1231.8 (s), 1185.1 (m), 1108.6 (w), 1055.9 (w), 997.2 (w), 903.0 (w), 845.3 (s), 806.2 (s), 764.1 (s), 662.3 (w). Anal. Calcd for C32H39N3CrCl3: C, 61.59; H, 6.30; N, 6.73. Found: C, 61.79; H, 6.03; N, 6.24.
Synthesis of 2-(1-(2,6-diisopropylphenylimino)ethyl)-7-(2,6-diisopropylphenylimino)-6,6-dimethylcyclopenta[b]pyridylchromium chloride (Cr3). In a manner similar to that described for Cr1, Cr3 was isolated as green powder 70.2% yield. FT-IR (KBr, cm−1): 2963.6 (m), 2922.9 (w), 2868.9 (w), 1648.7 (s), 1605.4 (m), 1589.0 (m), 1462.0 (s), 1433.9 (m), 1364.4 (s), 1323.3 (w), 1235.3 (w), 1184.1 (m), 1158.9 (w), 1110.4 (w), 1055.9 (w), 897.8 (m), 831.5 (w), 800.5 (m), 743.4 (w). Anal. Calcd for C36H47N3CrCl3: C, 63.57; H, 6.97; N, 6.18. Found: C, 63.91; H, 7.32; N, 6.14.
Synthesis of 2-(1-(2,4,6-trimethylphenylimino)ethyl)-7-(2,4,6-trimethylphenylimino)-6,6-dimethylcyclopenta[b]pyridylchromium chloride (Cr4). In a manner similar to that described for Cr1, Cr4 was isolated as green powder 76.1% yield. FT-IR (KBr, cm−1): 2967.5 (m), 2921.8 (w), 2871.5 (w), 1626.7 (s), 1601.5 (m), 1468.5 (m), 1376.3 (w), 1361.1 (m), 1320.3 (w), 1281.2 (w), 1194.0 (m), 1167.5 (w), 1137.8 (w), 1112.8 (w), 1020.3 (m), 953.0 (w), 891.2 (w), 848.9 (s), 774.6 (w), 665.3 (w). Anal. Calcd for C30H35N3CrCl3: C, 60.46; H, 5.92; N, 7.05. Found: C, 60.48; H, 5.77; N, 7.11.
Synthesis of 2-(1-(2,6-diethyl-4-methylphenylimino)ethyl)-7-(2,6-diethyl-4-methylphenylimino)-6,6-dimethylcyclopenta[b] pyridylchromium chloride (Cr5). In a manner similar to that described for Cr1, Cr5 was isolated as green powder 71.5% yield. FT-IR (KBr, cm−1): 2963.2 (w), 2913.5 (m), 2873.8 (w), 1627.3 (w), 1603.8 (m), 1571.0 (m), 1454.0 (s), 1419.7 (m), 1369.1 (m), 1311.4 (w), 1234.2 (w), 1194.7 (w), 1159.3 (w), 1110.3 (m), 1032.4 (m), 991.2 (m), 898.5 (w), 855.2 (s), 790.8 (w), 704.1 (w), 662.5 (w). Anal. Calcd for C34H43N3CrCl3: C, 62.62; H, 6.65; N, 6.44. Found: C, 62.34; H, 6.35; N, 6.14.
X-ray crystallographic studies
Single crystals of Cr1 suitable for X-ray diffraction analysis were obtained by slow diffusion of heptane into dichloromethane solution at room temperature. X-ray studies were carried out on a Rigaku Saturn724+CCD with graphite-monochromatic Mo Kα radiation (λ = 0.71073 Å) at 173(2) K; cell parameters were obtained by global refinement of the positions of all collected reflections. Intensities were corrected for Lorentz and polarization effects and empirical absorption. The structures were solved by direct methods and refined by full-matrix least squares on F2. All hydrogen atoms were placed in calculated positions. Structure solution and refinement were performed by using the SHELXL-97 package.35 Details of the X-ray structure determinations and refinements are provided in Table 5.
Table 5 Crystal data and structure refinement for Cr1
| Identification code |
Cr1 CH2Cl2 |
| Empirical formula |
C29H32Cl5CrN3 |
| Formula weight |
651.83 |
| Temperature/K |
173(2) |
| Wavelength/Å |
0.71073 |
| Crystal system |
Monoclinic |
| Space group |
P21/c |
| a/Å |
8.3300(17) |
| b/Å |
22.670(5) |
| c/Å |
16.960(3) |
| Alpha/° |
90 |
| Beta/° |
100.40 (3) |
| Gamma/° |
90 |
| Volume/Å3 |
3150.1(11) |
| Z |
4 |
| Dcalcd/(g cm−3) |
1.3744 |
| μ/mm−1 |
0.811 |
| F(000) |
1348.6 |
| Crystal size/mm |
0.175 × 0.086 × 0.059 |
| θ range/°C |
3.04–49 |
| Limiting indices |
−10 ≤ h ≤ 10 |
| −29 ≤ k ≤ 29 |
| −21 ≤ l ≤ 21 |
| No. of rflns collected |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746 746 |
| No. unique rflns [R(int)] |
5044(0.1184) |
| Completeness to θ |
96.1% |
| Data/restraints/parameters |
5044/0/349 |
| Goodness of fit on F2 |
1.044 |
| Final R indices [I > 2Σ(I)] |
R1 = 0.1035, wR2 = 0.1910 |
| R Indices (all data) |
R1 = 0.1164, wR2 = 0.1987 |
| Largest diff. peak and hole (e Å−3) |
0.62 and −0.60 |
Acknowledgements
This work was supported by National Natural Science Foundation of China (Nos. U1362204, 21473160, and 21374123).
Notes and references
-
(a) S. D. Ittel, L. K. Johnson and M. Brookhart, Chem. Rev., 2000, 100, 1169–1203 CrossRef CAS PubMed;
(b) V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283–315 CrossRef CAS PubMed;
(c) S. Mecking, Angew. Chem., Int. Ed., 2001, 40, 534–540 CrossRef CAS;
(d) D. S. McGuinness, Chem. Rev., 2011, 111, 2321–2341 CrossRef CAS PubMed;
(e) T. Agapie, Coord. Chem. Rev., 2011, 255, 861–880 CrossRef CAS.
-
(a) K. H. Theopold, Eur. J. Inorg. Chem., 1998, 1, 15–24 CrossRef;
(b) L. A. MacAdams, G. P. Buffone, C. D. Incarvito, A. L. Rheingold and K. H. Theopold, J. Am. Chem. Soc., 2005, 127, 1082–1083 CrossRef CAS PubMed;
(c) A. K. Tomov, J. J. Chirinos, D. J. Jones, R. J. Long and V. C. Gibson, J. Am. Chem. Soc., 2005, 127, 10166–10167 CrossRef CAS PubMed;
(d) F. Junges, M. C. A. Kuhn, A. H. D. P. dos Santos, C. R. K. Rabello, C. M. Thomas, J. F. Carpentier and O. L. Casagrande, Organometallics, 2007, 26, 4010–4014 CrossRef CAS;
(e) K. Albahily, D. Al-Baldawi, S. Gambarotta, E. Koç and R. Duchateau, Organometallics, 2008, 27, 5943–5947 CrossRef CAS;
(f) I. Vidyaratne, G. B. Nikiforov, S. I. Gorelsky, S. Gambarotta, R. Duchateau and I. Korobkov, Angew. Chem., Int. Ed., 2009, 48, 6552–6556 CrossRef CAS PubMed.
- A. Clark, Catal. Rev., 1969, 3, 145–173 CAS.
-
(a) J. P. Hogan, J. Polym. Sci., Part A: Polym. Chem., 1970, 8, 2637–2652 CrossRef CAS;
(b) F. J. Karol, G. L. Karapinka, C. Wu, A. W. Dow, R. N. Johnson and W. L. Carrick, J. Polym. Sci., Part A: Polym. Chem., 1972, 10, 2621–2637 CrossRef CAS.
- E. Groppo, C. Lamberti, S. Bordiga, G. Spoto and A. Zecchina, Chem. Rev., 2005, 105, 115–183 CrossRef CAS PubMed.
-
(a) T. J. Pullukat and R. E. Hoff, Catal. Rev. Sci. Eng., 1999, 41, 389–428 CrossRef CAS;
(b) I. Matos and Y. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 3464–3472 CrossRef CAS;
(c) B. Rebenstorf and J. Polym, J. Polym. Sci., Part A: Polym. Chem., 1991, 29, 1949–1953 CrossRef CAS;
(d) S. Zhang, Q. Dong, R. Cheng, X. He, Q. Wang, Y. Tang, Y. Yu, K. Xie, J. Da and B. Liu, J. Mol. Catal. A: Chem., 2012, 358, 10–22 CrossRef CAS;
(e) K. Y. Choi and S. Tang, J. Appl. Polym. Sci., 2004, 91, 2923–2927 CrossRef CAS;
(f) K. Y. Choi, S. Tang and W. Yoon, Macromol. Theory Simul., 2004, 13, 169–177 CrossRef CAS;
(g) Y. V. Kissin, A. J. Brandolini and J. L. Garlick, J. Polym. Sci. Part A: Polym. Chem., 2008, 46, 5315–5329 CrossRef CAS;
(h) P. C. Thune, R. Linke, W. J. H. van Geimip, A. M. de Jong and J. W. Niemantsverdriet, J. Phys. Chem. B, 2001, 105, 3073–3078 CrossRef;
(i) B. Liu, H. Nakatani and M. Terano, J. Mol. Catal. A: Chem., 2003, 201, 189–197 CrossRef CAS;
(j) B. Liu, Y. Fang, K. Hasebe and M. Terano, J. Mol. Catal. A: Chem., 2005, 238, 142–150 CrossRef CAS;
(k) B. Liu, H. Nakatani and M. Terano, J. Mol. Catal. A: Chem., 2002, 184, 387–398 CrossRef CAS;
(l) Y. Qi, Q. Dong, L. Zhong, Z. Liu, P. Qiu, R. Cheng, X. He, J. Vanderbilt and B. Liu, Organometallics, 2010, 29, 1588–1602 CrossRef CAS;
(m) P. Qiu, R. Cheng, B. Liu, B. Tumanskii, R. J. Batrice, M. Botoshansky and M. S. Eisen, Organometallics, 2011, 30, 2144–2148 CrossRef CAS;
(n) E. Groppo, C. Lamberti, S. Bordiga, G. Spoto and A. Zecchina, Chem. Rev., 2005, 105, 115–183 CrossRef CAS PubMed.
- D. Gong, W. Liu, T. Chen, Z.-R. Chen and K.-W. Huang, J. Mol. Catal. A: Chem., 2014, 395, 100–107 CrossRef CAS.
- S. Mark, H. Wadepohl and M. Enders, J. Org. Chem., 2016, 12, 1372–1379 Search PubMed.
-
(a) J. O. Moulin, J. Evans, D. S. McGuinness, G. Reid, A. J. Rucklidge, R. P. Tooze and M. Tromp, Dalton Trans., 2008, 1177–1185 RSC;
(b) D. F. Wass, Dalton Trans., 2007, 816–819 RSC;
(c) D. S. McGuinness, A. J. Rucklidge, R. P. Tooze and A. M. Slawin, Organometallics, 2007, 26, 2561–2569 CrossRef CAS;
(d) B. L. Small, M. J. Carney, D. M. Holman, C. E. O'Rourke and J. A. Halfen, Macromolecules, 2004, 37, 4375–4386 CrossRef CAS;
(e) I. Vidyaratne, J. Scott, S. Gambarotta and R. Duchateau, Organometallics, 2007, 26, 3201–3211 CrossRef CAS;
(f) W. Zhang, W.-H. Sun, X. Tang, T. Gao, S. Zhang, P. Hao and J. Chen, J. Mol. Catal. A: Chem., 2007, 265, 159–166 CrossRef CAS;
(g) H. Sugiyama, G. Aharonian, S. Gambarotta, G. P. Yap and P. H. Budzelaar, J. Am. Chem. Soc., 2002, 124, 12268–12274 CrossRef CAS PubMed.
-
(a) V. C. Gibson, S. Mastroianni, C. Newton, C. Redshaw, G. A. Solan, A. J. P. White and D. J. Williams, J. Chem. Soc., Dalton Trans., 2000, 1969–1971 RSC;
(b) V. C. Gibson, C. Newton, C. Redshaw, G. A. Solan, A. J. P. White and D. J. Williams, J. Chem. Soc., Dalton Trans., 1999, 827–829 RSC.
-
(a) L. A. MacAdams, G. P. Buffone, C. D. Incarvito, A. L. Rheingold and K. H. Theopold, J. Am. Chem. Soc., 2005, 127, 1082–1083 CrossRef CAS PubMed;
(b) W.-K. Kim, M. J. Fevola, L. M. Liable-Sands, A. L. Rheingold and K. H. Theopold, Organometallics, 1998, 17, 4541–4543 CrossRef CAS.
-
(a) A. Bollmann, K. Blann, J. T. Dixon, F. M. Hess, E. Killian, H. Maumela, D. S. McGuinness, D. H. Morgan, A. Nveling, S. Otto, M. Overett, A. M. Z. Slawin, P. Wasserscheid and S. Kuhlmann, J. Am. Chem. Soc., 2004, 126, 14712–14713 CrossRef CAS PubMed;
(b) T. Agapie, M. W. Day, L. M. Henling, J. A. Labinger and J. E. Bercaw, Organometallics, 2006, 25, 2733–2742 CrossRef CAS.
- Z. Hao, B. Xu, W. Gao, Y. Han, G. Zeng, J. Zhang, G. Li and Y. Mu, Organometallics, 2015, 34, 2783–2790 CrossRef CAS.
-
(a) R. D. Köhn, M. Haufe, G. Kociok-Köhn, S. Grimm, P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 4337–4339 CrossRef;
(b) R. D. Köhn, M. Haufe, S. Mihan and D. Lilge, Chem. Commun., 2000, 1927–1928 RSC;
(c) A. K. Tomov, J. J. Chirinos, R. J. Long, V. C. Gibson and M. R. J. Elsegood, J. Am. Chem. Soc., 2006, 128, 7704–7705 CrossRef CAS PubMed;
(d) Y. Chen, W. Zuo, P. Hao, S. Zhang, K. Gao and W.-H. Sun, J. Organomet. Chem., 2008, 693, 750–762 CrossRef CAS;
(e) L. Xiao, M. Zhang and W.-H. Sun, Polyhedron, 2010, 29, 142–147 CrossRef CAS.
- B. L. Small, M. J. Carney, D. M. Holman, C. E. O'Rourke and J. A. Halfen, Macromolecules, 2004, 37, 4375–4386 CrossRef CAS.
- R. J. Baker and P. G. Edwards, J. Chem. Soc., Dalton Trans., 2002, 2960–2965 RSC.
-
(a) A. Carter, S. A. Cohen, N. A. Cooley, A. Murphy, J. Scutt and D. F. Wass, Chem. Commun., 2002, 858–859 RSC;
(b) M. E. Bluhm, O. Walter and M. Döring, J. Organomet. Chem., 2005, 690, 713–721 CrossRef CAS.
- D. S. McGuinness, P. Wasserscheid, W. Keim, D. Morgan, J. T. Dixon, A. Bollmann, H. Maumela, F. Hess and U. Englert, J. Am. Chem. Soc., 2003, 125, 5272–5273 CrossRef CAS PubMed.
- D. S. McGuinness, V. C. Gibson, D. F. Wass and J. W. Steed, J. Am. Chem. Soc., 2003, 125, 12716–12717 CrossRef CAS PubMed.
- J. Liu, Y. Li, J. Liu and Z. Li, J. Mol. Catal. A: Chem., 2006, 244, 99–104 CrossRef CAS.
-
(a) M. A. Esteruelas, A. M. López, L. Méndez, M. Oliván and E. Oñate, Organometallics, 2003, 22, 395–406 CrossRef CAS;
(b) Y. Nakayama, K. Sogo, H. Yasuda and T. Shiono, J. Polym. Sci. Part A: Polym. Chem., 2005, 43, 3368–3375 CrossRef CAS.
-
(a) W. Zhang, W. Chai, W.-H. Sun, X. Hu, C. Redshaw and X. Hao, Organometallics, 2012, 31, 5039–5048 CrossRef CAS;
(b) W.-H. Sun, S. Kong, W. Chai, T. Shiono, C. Redshaw, X. Hu, C. Guo and X. Hao, Appl. Catal., A, 2012, 67, 447–448 Search PubMed.
- V. K. Appukuttan, Y. Liu, B. C. Son, C. Ha, H. Suh and I. Kim, Organometallics, 2011, 30, 2285–2294 CrossRef CAS.
-
(a) F. Huang, Q. Xing, T. Liang, Z. Flisak, B. Ye, X. Hu, W. Yang and W.-H. Sun, Dalton Trans., 2014, 43, 16818–116829 RSC;
(b) F. Huang, W. Zhang, E. Yue, T. Liang, X. Hu and W.-H. Sun, Dalton Trans., 2016, 45, 657–666 RSC.
- J. Ba, S. Du, E. Yue, X. Hu, Z. Flisak and W.-H. Sun, RSC Adv., 2015, 5, 32720–32729 RSC.
- J.-H. So and P. Boudjouk, Inorg. Chem., 1990, 29, 1592–1593 CrossRef CAS.
-
(a) M. Zhang, K. Wang and W.-H. Sun, Dalton Trans., 2009, 6354–6363 RSC;
(b) S. Zhang, S. Jie, Q. Shi and W.-H. Sun, J. Mol. Catal. A: Chem., 2007, 276, 174–183 CrossRef CAS;
(c) A. A. Saliu, A. Maliha, M. Zhang, G. Li and W.-H. Sun, Aust. J. Chem., 2008, 61, 397–403 CrossRef;
(d) R. Gao, T. Liang, F. Wang and W.-H. Sun, J. Organomet. Chem., 2009, 694, 3701–3707 CrossRef CAS.
-
(a) J. Yu, H. Liu, W. Zhang, X. Hao and W.-H. Sun, Chem. Commun., 2011, 47, 3257–3259 RSC;
(b) J. Lai, W. Zhao, W. Yang, C. Redshaw, T. Liang and W.-H. Sun, Polym. Chem., 2012, 3, 787–793 RSC;
(c) W.-H. Sun, W. Zhao, J. Yu, W. Zhang, X. Hao and C. Redshaw, Macromol. Chem. Phys., 2012, 213, 1266–1273 CrossRef CAS;
(d) W. Zhao, J. Yu, S. Song, W. Yang, H. Liu, X. Hao, C. Redshaw and W.-H. Sun, Polymer, 2012, 53, 130–137 CrossRef CAS;
(e) X. Cao, F. He, W. Zhao, Z. Cai, X. Hao, T. Shiono, C. Redshaw and W.-H. Sun, Polymer, 2012, 53, 1870–1880 CrossRef CAS;
(f) S. Wang, W. Zhao, X. Hao, B. Li, C. Redshaw, Y. Li and W.-H. Sun, J. Organomet. Chem., 2013, 731, 78–84 CrossRef CAS;
(g) S. Wang, B. Li, T. Liang, C. Redshaw, Y. Li and W.-H. Sun, Dalton Trans., 2013, 42, 9188–9197 RSC.
-
(a) J. Yu, Y. Zeng, W. Huang, X. Hao and W.-H. Sun, Dalton Trans., 2011, 40, 8436–8443 RSC;
(b) S. A. Svejda and M. Brookhart, Organometallics, 1999, 18, 65–74 CrossRef CAS.
- S. Du, S. Kong, Q. Shi, J. Mao, C. Guo, J. Yi, T. Liang and W.-H. Sun, Organometallics, 2015, 34, 582–590 CrossRef CAS.
- W. Zhang, W.-H. Sun, S. Zhang, J. Hou, K. Wedeking, S. Schultz, R. Fröhlich and H. Song, Organometallics, 2006, 25, 1961–1969 CrossRef CAS.
-
(a) J.-Y. Liu, Y. Zheng, Y.-G. Li, L. Pan, Y.-S. Li and N.-H. Hu, J. Organomet. Chem., 2005, 690, 1233–1239 CrossRef CAS;
(b) W.-H. Sun, P. Hao, G. Li, S. Zhang, W. Wang, J. Yi, M. Asma and N. Tang, J. Organomet. Chem., 2007, 692, 4506–4518 CrossRef CAS.
-
(a) W.-H. Sun, S. Zhang, S. Jie, W. Zhang, Y. Li, H. Ma, J. Chen, K. Wedeking and R. Fröhlich, J. Organomet. Chem., 2006, 691, 4196–4203 CrossRef CAS;
(b) L. Xiao, R. Gao, M. Zhang, Y. Li, X. Cao and W.-H. Sun, Organometallics, 2009, 28, 2225–2233 CrossRef CAS.
-
(a) Q. Xing, T. Zhao, S. Du, W. Yang, T. Liang, C. Redshaw and W.-H. Sun, Organometallics, 2014, 33, 1382–1388 CrossRef CAS;
(b) M. Yankey, C. Obuah and J. Darkwa, Macromol. Chem. Phys., 2014, 215, 1767–1775 CrossRef CAS.
- G. M. Sheldrick, SHELXTL-97, Program for the Refinement of Crystal Structures, University of Göttingen, Germany, 1997 Search PubMed.
Footnote |
| † CCDC 1502005 contain the supplementary crystallographic data for complex Cr1. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra21914d |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.  *bcd
*bcd
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) stretching vibrations into a lower field, illustrating the effective coordination between the imino-nitrogen and chromium atom. Moreover, their elemental analyses are consistent with Cr1–Cr5. In addition, the molecular structure of the complexes Cr1 was confirmed by single-crystal X-ray diffraction studies.
N) stretching vibrations into a lower field, illustrating the effective coordination between the imino-nitrogen and chromium atom. Moreover, their elemental analyses are consistent with Cr1–Cr5. In addition, the molecular structure of the complexes Cr1 was confirmed by single-crystal X-ray diffraction studies.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746
746
