Nickel concentration-dependent opto-electrical performances and stability of Cu@CuNi nanowire transparent conductors†
Jie Xue,
Jizhong Song*,
Yousheng Zou,
Chengxue Huo,
Yuhui Dong,
Leimeng Xu,
Jianhai Li and
Haibo Zeng*
Institute of Optoelectronics & Nanomaterials, Herbert Gleiter Institute of Nanoscience, School of Materials Science and Engineering, Nanjing University of Science & Technology, Nanjing 210094, China. E-mail: songjizhong@njust.edu.cn; zeng.haibo@njust.edu.cn
First published on 13th September 2016
Abstract
Compared to monometallic counterparts, core–shell structured nanowires may possess additional performances or even new properties because of synergistic effects between two components. Particularly, the alloying shell may bring the advantage that we can adjust its components and sizes to achieve the desired performance. Here, we mainly study Ni-dependent electric stability and opto-electrical performances of Cu@CuNi NW electrodes. And we find that the increase of nickel content has little effect on optical performance, but effectively improves the stability of Cu@CuNi NW electrodes. We can also achieve ideal performance through regulating the nickel content and outstanding performance may come from the particular structure. Hence we studied Ni-dependent microstructure variations of Cu@CuNi NWs with the corresponding properties. The high stability NWs with a high nickel content have smooth CuNi alloying shells which effectively protect the NWs from oxidation. At the same time, the large length diameter ratio and high degree of crystallinity of Cu@CuNi NWs are the reasons for good transparent conductive properties. These demonstrations of controlling the composition of alloying shells for oxidation resistance of the NWs could bring forth great opportunities for transparent, flexible, stretchable, and wearable electronic and optoelectronic devices.
Introduction
Recently, metallic core–shell nanostructures have been a hot topic due to their amazing performance in a lot of fields, such as catalysis,1–3 transparent conductors,4 nanoelectronics,5 microwave absorption,6 chemical sensing7 and magnetic resonance imaging (MRI) enhancement.8,9 Among these nanostructures, the core–shell structured metallic nanowires (NWs) are particularly interesting. Compared with monometallic NWs, the microstructure and composition of core–shell structured NWs are different along the radial direction so that the synergistic effects between different components play an important role in improving the performance of them. Thus, many studies have been done on the synthesis, structural characterization and property exploration of core–shell structured metallic NWs,5,10–12 especially for the NWs with great potential for applications. For example, because of their low cost and abundance, Cu NWs have huge potential as alternatives to indium tin oxide (ITO)13–15 and Ag NWs16–20 in the future practical use of transparent electrodes (TEs),15 which are widely used as a key component in many applications, including touch screens, smart phones, e-readers, flat panel displays, organic solar cells, organic light-emitting diodes, and energy conversion and storage devices.21–25 However, the lack of stable conductivity in Cu NW TEs, due to a higher sensitivity to oxygen and moisture than other TE materials, is a serious bottleneck for their future practical use. Wiley et al. demonstrated an effective chemical solution process to add Ni shells on the surface of as-synthesized Cu NWs to improve the oxidation-resistant stability of the Cu NWs.4 However, two different materials as well as two separate steps may cause a discontinuous core–shell interface of the copper nanowires, poor crystallization and a rough surface. Meanwhile, Song et al. proposed a scale-up one-pot method to synthesize Cu NWs with copper–nickel alloying shells to protect Cu NWs from oxidation.26 However, reports on the influence of changing the components of the Cu–Ni alloy shells on the oxidation-resistant behavior, as well as the optical and electrical performance, are very rare. Thus, it's necessary and urgent to do research about this in order to provide a strong theoretical and experimental foundation for the excellent performance and for future industrialized applications.Here, we mainly study Ni-dependent electric stability and opto-electrical performances of Cu@CuNi NW electrodes. And we find that the increase of nickel content has little effect on the optical performance, but effectively improves the stability of the Cu@CuNi NW electrodes which can be used for more than 55 years. We can also achieve ideal performance through regulating the nickel content. It is well known that good performance comes from good structure. Hence we studied the microstructures of Cu@CuNi NWs with the corresponding properties. Then, the reason for the good performance was found to be that the high stability NWs with high nickel content have smooth Cu–Ni alloying shells, which effectively protect the NWs from oxidation. At the same time, the large length diameter ratio and high degree of crystallinity of the Cu@CuNi NWs are the reasons for the good transparent conductive properties (30 ohm per sq. at 80–90% transparency). In summary, these demonstrations of controlling the composition of alloying shells for oxidation resistance of the NWs could bring forth great opportunities for transparent, flexible, stretchable, and wearable electronic and optoelectronic devices.
Experimental section
1 Synthesis
 | ||
| Fig. 1 Ni-dependent microstructure variations of Cu@CuNi NWs. (a) Photograph of large-yield Cu NW inks re-dispersed in hexane, coated by a shell with different nickel content, 0, 3.4, 5.1, 9.6, and 17.2 at% (nickel content here means the atomic ratio of nickel in the entire nanowire), respectively. (b–f) TEM images of Cu NWs with different nickel content, 0, 3.4, 5.1, 9.6, and 17.2 at% respectively. (g) Plot of shell thickness (black line) and shell nickel content (blue line) of NWs vs. nickel content. Error bars show one standard deviation for five measurements. The inset of (g) shows schematic illustrations of the Cu–Ni alloy shell growth process, and the ratio of copper and nickel in the shell is illustrated in the ESI.† | ||
2 Materials characterization
The micro morphology of the NWs was obtained by a scanning electron microscope (SEM) (FEI Quant 250FEG) instrument. For Transmission Electron Microscopy (TEM), High Resolution Transmission Electron Microscopy (HRTEM) and Selected Area Electron Diffraction (SAED), a copper grid was used to hold the NWs, which were detected by a TECNAI G2 20 LaB6 instrument operated at an acceleration voltage of 200 kV. X-ray diffraction (XRD) patterns were acquired using a Bruker-AXS D8 Advance XRD instrument operating with Cu Kα radiation (λ = 1.5406 Å). Ultraviolet-visible spectroscopy (UV/Vis) transmission spectra were obtained using a Shimadzu 3600 UV/Vis spectrophotometer (in the 250–800 nm spectral range). The sheet resistance of the NW films was obtained via four-point probe measurements and the van der Pauw method at room temperature, and the final values were averaged over a minimum of five measurements for each sample.Results and discussion
1 The influence of different nickel content shells on morphology and structure
The detailed NW microstructure with different nickel content fabricated via a one-pot heating method was analysed by TEM as shown in Fig. 1(b–f). As shown in Fig. 1(b), the pure copper NWs have an average diameter of 20 nm and smooth surfaces. The diameter of the Cu NWs with 3.4 at% Ni was increased to 23 nm, and the core–shell interface is fuzzy (Fig. 1(c)). When the Ni content was increased to 5.1 at%, 9.6 at% and 17.2 at%, the interface became very clear with shell thicknesses of 2.1 nm, 5 nm and 6.5 nm, respectively (Fig. 1(d–f)). The diameter of the alloying Cu NWs clearly increased along with the Ni content, as observed in the TEM images. The diameter of the alloying Cu NWs can reach 53 nm, which may degrade the light transmission due to less void among the NWs. And on the basis of the Cu-to-Ni atomic ratio and core-to-shell volume ratio, the composition of the alloyed shell can be deduced as shown in Fig. 1(g). However, the increase of the diameter of the NWs does not affect their high aspect ratios (>1000), as shown in Fig. S2,† which will favor their excellent conductivity due to the resulting long and smooth transport channels for electrons.The as-synthesized NWs also have a high flexibility after adding the alloy shells, and can be bent to form a circle (Fig. S1†). From the SEM images (Fig. S3†), the Ni content on the Cu NW shells can be effectively controlled, and has little effect on the NW morphology without any particle-like products which were usually unavoidable in previous studies and significantly depressed the transparency and conductivity.4
Fig. 2(a) clearly shows that the alloy coated Cu NW with a diameter of 40 nm has a very smooth and clean surface. The thickness of the core and shell is 30 nm and 5 nm, respectively and the composition of the alloyed shell can be expressed as Cu4Ni. The [111] twin planar spacing of 0.206 nm of both the core and shell shown in Fig. 2(b) confirms that the growth of the NW is along the 〈110〉 direction. Generally, Cu NWs have a 5-fold twinned crystal structure bounded by 5 (100) side planes and 10 well-resolved (111) lattice fringes, similar to silver NWs, and the TEM cross section of a microtomed core–shell NW showing the 5-fold twinned crystal structure is given in Fig. 2(c).28,29 Fig. 2(d) shows the SAED patterns of the core–shell NWs which are found to be a superimposition of two face centered cubic patterns corresponding to zone [001] generated from T1 and zone [112] from T3 and T4. This can be attributed to the double-diffraction effect through the twin boundaries and sub-crystals, when the electron beam is perpendicular to one side of the surfaces of the NW (0°), marked in the inset of Fig. 2(c).
The XRD patterns directly verify that surface alloying can effectively change the structure of NW shells (Fig. S3†). A small but obvious Cu2O (111) diffraction peak appears at 36.8° (JCPDS no 05-0667) after exposing the Cu NW films to air during the preparation and characterization of Cu NW films. The diffraction peak (51°) of core–shell NW films on glass spilts apart slightly, directly confirming the formation of the copper nickel alloy phase by JCPDS no 09-0205.4,30
2 The influence of shell nickel content on optical and electrical performance
To analyse the effect of nickel content on opto-electrical properties, a simple process was used to fabricate NW films. First, the surface of the glass should be cleaned ultrasonically 3 times with acetone, and dried with nitrogen. Next, NWs were dispersed in hexane with a concentration of ∼0.02 mg mL−1 and filtered onto a nitrocellulose filter membrane.26 The amount of NWs collected on the membrane was used to control the NW film density and optical transmission. After filtration, the filter membranes were transferred onto the required substrates, such as polyimide (PI), polyethylene terephthalate (PET) and PDMS. Finally, the NW films were heat-treated at 200 °C for 1 h in an atmosphere of 95% Ar and 5% H2 to remove the resident oleylamine molecules on the surface of the NWs.The relationship between the transmittance as well as sheet resistance and the nickel content under the same density of NW films are shown in Fig. 3(a). We can see that the increase of the diameter had a slight effect on the sheet resistance but to a certain extent reduced the transmittance by 13%. The slight decrease in transmittance is because the diameter of the NWs increased after being coated with nickel. Overall, the relationship between transmittance and sheet resistance when alloyed with different amounts of nickel for Cu NW films is shown in Fig. 3(c). The plot suggests that the transmittance of the NW network films at a given sheet resistance of 30 ohm per sq. decreases from 92% to 80% with increasing nickel content, consistent with Fig. 3(a). The alloying shell Cu NW films with 9.6 at% nickel show a sheet resistance of 30 ohm per sq. at 84% transparency. Compared to pure Cu NW films, the surface alloying behavior does not degrade transparency and conductivity. The alloying shell Cu NW films with excellent transmittance show an extremely low sheet resistance (below 100 ohm per sq.), shown in Fig. S4,† which well meets the industrial requirements and it is comparable to the best performance of Ag NW network electrodes.31,32 What's more, the alloying shell Cu NW network films shown in the large-scale photograph (Fig. 3(b)), demonstrated the compatibility of copper NWs with flexible substrates and exhibited excellent transparency over almost all of the visible range, and had a sheet resistance of 30 ± 3 ohm per sq. over the whole area.
In order to obtain comprehensive characterization of transparent conductive properties more accurately, the transmittance at 550 nm was plotted as a function of the sheet resistance for all Cu NW films studied in this work, as shown in Fig. 3(c). The relation between the transmittance (T) and the sheet resistance (Rs) of Cu NW thin films was quantitatively analyzed using eqn (1)33
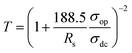 | (1) |
3 The influence of shell nickel content on the oxidation-resistant behavior
As described in the introduction, the main factor that restricts Cu NW application in various optoelectronic devices is its poor oxidation-resistance in the air, because Cu is much more easily oxidized than Ag and Au by oxygen and moisture in the natural atmosphere. The highly crystalline alloyed shells were used to enhance the electrical stability of Cu NW network films.The effects of different nickel content on the electrical stability of Cu NW films are indirectly shown in Fig. 4(a). Our experiments were conducted under ambient conditions (at the temperature of 25 °C). We recorded the experimental environment moisture level. The environment moisture level changed from 45% to 70% due to the coming rainy season. The sheet resistance of the pure Cu NW films with a sheet resistance of 16 ohm per sq. at 85% transparency began to increase after 1 day and increased by an order of magnitude after 15 days. The stability is clearly improved after being shell alloyed with nickel (3.4 at%) as a protective layer, and the sheet resistance increased by 76% after 30 days. The sheet resistance of the core–shell NW film with 5.1 at% nickel maintained relatively high electrical stability over a period of 30 days, increasing by only 14 ohm per sq. (from 30 to 44 ohm per sq.). When the Ni content is increased to 9.6 at%, the formed NW films become completely impervious to oxygen and moisture. And the change of sheet resistance over 30 days is very small, within 2 ohm per sq., which is close to measurement error. Combined with the transmittance and the electrical stability of the alloying Cu NW films, the optimal Ni content is 9.6 at%, and these films have a sheet resistance of 40 ohm per sq. at a transmittance of 80%.
A doubling change in sheet resistance would not be suitable for most practical applications, so the analysis was limited to data for which ΔR/R0 < 1. All the extracted data for which ΔR/R0 < 1 can be best fitted with a linear rate law according to oxidation kinetics reflected by eqn (2).4,34
| ΔR/R0 = kt + C | (2) |
| ln(k) = −Ea/R(1/T) + ln(A) | (3) |
As we can see in Fig. 4(d), the lifetimes at which ΔR/R0 = 1 at room temperature (25 °C) were estimated using the Arrhenius plot and are 0.06, 16, 24, 55 and 66 years for the Cu NWs containing 0, 3.4, 5.1 and 9.6 at% Ni, respectively. As a consequence, the nickel alloying shell effectively improved the oxidation resistance of Cu NWs. Particularly, with Ni contents of 9.6 at% or greater, the life of NW films can be up to 50 years or more. The molar ratio of Ni to Cu of the alloyed shell can be deduced to be Cu4Ni.26 The enhancement of the electrical stability of Cu NWs by the Cu4Ni alloy layer is attributed to the particular nickel content in copper that can act as a dopant, and change the point defect concentration and govern the ion mobility.36 What's more, during the process of oxidation, the grain boundary is used as an oxidation channel to expand the oxidation process. Therefore the excellent crystalline and smooth surface of NWs also reduces the oxidation rate of diffusion. At the same time, a compact and uniform NiO layer is formed to protect copper to prevent oxidation.37 However, the conductivity of the membrane will be reduced when the NWs are coated with a high nickel content and that will cause a thicker nickel oxide layer. Therefore, considering the two aspects of electricity and stability, 9.6 at% is the best nickel content for NWs and the second best is 5.1 at% Ni content.
The enhancement of electrical stability of Cu NWs by the Cu4Ni alloy layer is attributed to the increase of nickel content, which greatly slows down the speed of oxidation of the copper NWs. For the two samples containing 5.1 at% Ni and 9.6 at% Ni, their transparent conductive performance is very excellent and the electrical stability is also good. Therefore we carried out in situ XRD detection for core–shell Cu NWs containing 5.1 at% Ni and 9.6 at% Ni to intuitively observe the phase transition of NWs in the process of oxidation at 130 °C in air and the results are shown in Fig. 5. In Fig. 5(a), we can see that diffraction peaks (200) and (111) (XRD spectra as shown in Fig. S16†) of the Cu NWs containing 5.1 at% Ni are getting smaller and smaller with increasing time. However, when the Ni content is increased to 9.6 at%, the diffraction peak (51°) of nickel and the Cu diffraction peaks (200) and (111) decrease relatively slowly, even when the heating time reached 340 minutes as shown in Fig. 5(b). The changes of the diffraction peak (200) can be intuitively plotted and presented in Fig. 5(c). Interestingly, at the end of the oxidation, no diffraction peaks of the CuO were formed, which indicated that the final oxidation products are in an amorphous state.
In order to explore the compositional changes of copper and nickel after oxidation, we performed X-ray photoelectron spectroscopy (XPS) on Cu containing 5.1 at% Ni and 9.6 at% Ni before and after heating as shown in Fig. 6 and S17.† As we can see in Fig. 6(a), the 2p3/2 signal of Ni(0) from Cu@Cu4Ni alloy NWs exhibits peaks at 853.5 eV. But there was no obvious Ni(0) peak in the full spectrum when the nickel content was 5.1 at%. The reason may be that the surface Ni content is too low to detect. In Fig. 6(b), XPS of the Cu NWs containing 5.1 at% Ni exhibits peaks at 932.7 and 952.4 eV, characteristic of the 2p3/2 and 2p1/2 binding energies of Cu(0) metal.38 After being heated at 130 °C for 80 minutes, the 2p peaks shift toward higher binding energy and additional rounded peaks from CuO appeared at 942.4 and 962.4 eV. Correspondingly, the normalized increase in the sheet resistance (ΔR/R0) of Cu NW films happens to be equal to 1. As we can see, Cu species present on the Cu NWs are not entirely cupric oxide when ΔR/R0 = 1. Thus, we can assume that the sheet resistance of copper NWs increases due to the oxidation of the copper NW’s intersection points (mainly) and the surface.
Under the same conditions, Cu NWs containing 9.6 at% Ni films were heated at 120 °C for 80 minutes. Fig. 6(c) shows that after coating Cu NWs with a thicker layer of Cu–Ni alloy, the signals for Cu(0) decrease by approximately 2 times. And there is no shift of the peaks of Cu toward higher binding energies after being heated, consistent with the fact that Cu NWs are protected from oxidation by Ni, which further confirms that the activity of the copper surface is decreased by the addition of nickel.
4 Alloying Cu NW TEs for optoelectronics
The core–shell NWs with high crystallinity, uniform morphology and mono-dispersity can be easily assembled into films by mature solution-based processes, such as a filtration method, spin coating, and spray coating. The solution process makes the NW inks readily deposited on a variety of substrates such as PI, PET, PDMS, and glass. The facile shape Cu NW electrodes were used as segments of external circuits for LEDs as shown in Fig. S18.† The working LED was operated at 2.2 V with various shape and material substrate NW films as conductors. The photos of the whole circuit are shown in Fig. S19.† The stability of the performances of the LED sufficiently proves that core–shell NW films have a promising potential for application in a variety of flexible and stretchable devices, especially new fields such as wearable electronics, robotic skins, electronic skins, and implantable medical devices.Conclusion
In summary, Cu–Ni alloying shell NWs were prepared via a one-pot method with unique virtues such as extremely crystalline alloyed shells, clear and abrupt interfaces, flat-surfaces, and a length more than 50 μm. Using these typical samples, we have firstly studied the effects of different nickel content on the transmittance, electrical properties, and oxidation-resistant behaviors by controlling the alloy composition. Despite the transmittance of the NW films showing a slight decrease, from 92% to 85% at a sheet resistance of 30 ohm per sq., the higher nickel content contributes to the stronger oxidation resistance of the copper NWs. We still verified the importance of the Cu–Ni alloying shell for oxidation resistance via XRD and XPS measurements. Importantly, when the nickel and copper ratio is about 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the NW shells, the stability of the alloying NW films can be improved, being 900 times more resistant to oxidation relative to pure Cu NWs. What's more, the oxidation-resistance of NW films with the nickel content up to 17.2% is similar to Cu@Cu4Ni NWs, but the NW samples have some by-products, leading to NW films with relatively lower transmittance. With the demonstration of a proven high performance and extremely high stability, these alloying Cu–Ni shell NW films have potential for application in a wide variety of flexible and stretchable devices, especially new fields such as wearable electronics, robotic skins, electronic skins, and implantable medical devices.
1 for the NW shells, the stability of the alloying NW films can be improved, being 900 times more resistant to oxidation relative to pure Cu NWs. What's more, the oxidation-resistance of NW films with the nickel content up to 17.2% is similar to Cu@Cu4Ni NWs, but the NW samples have some by-products, leading to NW films with relatively lower transmittance. With the demonstration of a proven high performance and extremely high stability, these alloying Cu–Ni shell NW films have potential for application in a wide variety of flexible and stretchable devices, especially new fields such as wearable electronics, robotic skins, electronic skins, and implantable medical devices.
Acknowledgements
This work was financially supported by the National Basic Research Program of China (2014CB931700/2014CB931702), The National Key Research and Development Program of China (2016YFB0401700/2016YFB0401701), NSFC (51572128, 61604074), NSFC-RGC (5151101197), the Natural Science Foundation of Jiangsu Province (BK20160827), China Postdoctoral Science Foundation (2016M590455), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- D. Wang and Y. Li, Adv. Mater., 2011, 23, 1044–1060 CrossRef CAS PubMed.
- J.-M. Yan, X.-B. Zhang, T. Akita, M. Haruta and Q. Xu, J. Am. Chem. Soc., 2010, 132, 5326–5327 CrossRef CAS PubMed.
- H.-L. Jiang, T. Akita and Q. Xu, Chem. Commun., 2011, 47, 10999–11001 RSC.
- A. R. Rathmell, M. Nguyen, M. Chi and B. J. Wiley, Nano Lett., 2012, 12, 3193–3199 CrossRef CAS PubMed.
- D. Feldheim and C. Keating, Chem. Soc. Rev., 1998, 27, 1–12 RSC.
- J. Guo, X. Wang, P. Miao, X. Liao, W. Zhang and B. Shi, J. Mater. Chem., 2012, 22, 11933–11942 RSC.
- P. Sudeep, S. S. Joseph and K. G. Thomas, J. Am. Chem. Soc., 2005, 127, 6516–6517 CrossRef CAS PubMed.
- J. Cheon and J.-H. Lee, Acc. Chem. Res., 2008, 41, 1630–1640 CrossRef CAS PubMed.
- C. Sun, J. S. Lee and M. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1252–1265 CrossRef CAS PubMed.
- Z. Liu, D. Elbert, C.-L. Chien and P. C. Searson, Nano Lett., 2008, 8, 2166–2170 CrossRef CAS PubMed.
- M. McKiernan, J. Zeng, S. Ferdous, S. Verhaverbeke, K. S. Leschkies, R. Gouk, C. Lazik, M. Jin, A. L. Briseno and Y. Xia, Small, 2010, 6, 1927–1934 CrossRef CAS PubMed.
- C. M. Bowers, A. A. Shestopalov, R. L. Clark and E. J. Toone, ACS Appl. Mater. Interfaces, 2012, 4, 3932–3937 CAS.
- P. Sajanlal and T. Pradeep, Nanoscale, 2012, 4, 3427–3437 RSC.
- Y.-C. Lai, Y.-C. Huang, T.-Y. Lin, Y.-X. Wang, C.-Y. Chang, Y. Li, T.-Y. Lin, B.-W. Ye, Y.-P. Hsieh and W.-F. Su, NPG Asia Mater., 2014, 6, e87 CrossRef CAS.
- B. Azzopardi, C. J. Emmott, A. Urbina, F. C. Krebs, J. Mutale and J. Nelson, Energy Environ. Sci., 2011, 4, 3741–3753 Search PubMed.
- L. Hu, H. S. Kim, J.-Y. Lee, P. Peumans and Y. Cui, ACS nano, 2010, 4, 2955–2963 CrossRef CAS PubMed.
- D. Angmo, T. R. Andersen, J. J. Bentzen, M. Helgesen, R. R. Søndergaard, M. Jørgensen, J. E. Carlé, E. Bundgaard and F. C. Krebs, Adv. Funct. Mater., 2015, 25, 4539–4547 CrossRef CAS.
- J. H. Yim, S.-y. Joe, C. Pang, K. M. Lee, H. Jeong, J.-Y. Park, Y. H. Ahn, J. C. de Mello and S. Lee, ACS Nano, 2014, 8, 2857–2863 CrossRef CAS PubMed.
- M. Amjadi, A. Pichitpajongkit, S. Lee, S. Ryu and I. Park, ACS Nano, 2014, 8, 5154–5163 CrossRef CAS PubMed.
- J. Kim, S. H. Lee, H. Kim, S. H. Kim and C. E. Park, ACS Appl. Mater. Interfaces, 2015, 7, 14272–14278 CAS.
- L. Gomez De Arco, Y. Zhang, C. W. Schlenker, K. Ryu, M. E. Thompson and C. Zhou, ACS Nano, 2010, 4, 2865–2873 CrossRef CAS PubMed.
- R. G. Gordon, MRS Bull., 2000, 25, 52–57 CrossRef CAS.
- J. Song and H. Zeng, Angew. Chem., Int. Ed., 2015, 54, 9760–9774 CrossRef CAS PubMed.
- J. Song, S. A. Kulinich, J. Li, Y. Liu and H. Zeng, Angew. Chem., 2015, 127, 472–476 CrossRef.
- J. Song and H. Zeng, Angew. Chem., 2015, 127, 9896–9910 CrossRef.
- J. Song, J. Li, J. Xu and H. Zeng, Nano Lett., 2014, 14, 6298–6305 CrossRef CAS PubMed.
- H. Guo, Y. Chen, H. Ping, J. Jin and D.-L. Peng, Nanoscale, 2013, 5, 2394–2402 RSC.
- H.-J. Yang, S.-Y. He and H.-Y. Tuan, Langmuir, 2014, 30, 602–610 CrossRef CAS PubMed.
- B. Wiley, Y. Sun and Y. Xia, Acc. Chem. Res., 2007, 40, 1067–1076 CrossRef CAS PubMed.
- W. Liu, Y. Fang, Y. Xu, X. Li and L. Li, Sci. China: Technol. Sci., 2014, 57, 2536–2541 CrossRef.
- P.-C. Hsu, D. Kong, S. Wang, H. Wang, A. J. Welch, H. Wu and Y. Cui, J. Am. Chem. Soc., 2014, 136, 10593–10596 CrossRef CAS PubMed.
- F. Xu and Y. Zhu, Adv. Mater., 2012, 24, 5117–5122 CrossRef CAS PubMed.
- F. Mirri, A. W. Ma, T. T. Hsu, N. Behabtu, S. L. Eichmann, C. C. Young, D. E. Tsentalovich and M. Pasquali, ACS Nano, 2012, 6, 9737–9744 CrossRef CAS PubMed.
- A. Yabuki and S. Tanaka, Mater. Res. Bull., 2011, 46, 2323–2327 CrossRef CAS.
- J. Castle and M. Nasserian-Riabi, Corros. Sci., 1975, 15, 537–543 CrossRef CAS.
- J. Li, J. Mayer and E. Colgan, J. Appl. Phys., 1991, 70, 2820–2827 CrossRef CAS.
- F. Wang, Oxid. Met., 1997, 47, 247–258 CrossRef CAS.
- C. D. Wagner, Handbook of x-ray photoelectron spectroscopy: a reference book of standard data for use in x-ray photoelectron spectroscopy, physical electronics division, Perkin-Elmer Corp., 1979 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental synthesis procedures, characterization, including additional UV-vis, TEM, SEM, Cu–Ni content analysis, and plot of ΔR/R0 vs. time for copper NW films, Cu NWs embedded in polymer, and transparent and conductive elastomer samples, are presented. See DOI: 10.1039/c6ra19577f |
| This journal is © The Royal Society of Chemistry 2016 |





