Transition metal based single chained surfactants: synthesis, aggregation behavior and enhanced photoluminescence properties of fluorescein†
Preeti Garg,
Gurpreet Kaur* and
Ganga Ram Chaudhary*
Department of Chemistry, Centre of Advanced Studies in Chemistry, Panjab University, Chandigarh, India. E-mail: gurpreet14@pu.ac.in; grc22@pu.ac.in; Fax: +91-172-2545074; Tel: +91-172-2534431 Tel: +91-172-2534406
First published on 21st October 2016
Abstract
Four different metal based surfactants were synthesized using hexadecyltrimethyl ammonium chloride and metal chlorides (FeCl2, CoCl2, NiCl2 and CuCl2) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios. The prepared metal complexes were characterized by different techniques such as FTIR, CHNS elemental analysis, atomic absorption spectrophotometry and NMR. The effect of metal counter ion on the thermal and aggregation behaviour of CTAC was studied by TGA, conductivity and surface tension. The results reveal that metal incorporation decreases the CMC value and increases the thermal stability in the order Cu(II) > Co(II) > Ni(II) > Fe(II). The study of absorption and fluorescence spectra of fluorescein in pre and post micellar concentration of metallosurfactants was carried out. The binding constant and number of binding sites was evaluated by using Stern–Volmer equation. Fluorescence quantum yield (ΦF) of fluorescein (FL) was premeditated in prepared metallosurfactants (FeC I, CoC I, NiC I, CuC I). This single alkyl chain system was used to gain reliable insight into the contribution of metal towards the ΦF of FL. The analysis of results indicate that among all, copper surfactant exhibited high fluorescence quantum yield due to increase in the radiative decay of FL. Even as the concentration of metallosurfactant goes from pre-micellar to post-micellar, the ΦF of FL was increased.
1 ratios. The prepared metal complexes were characterized by different techniques such as FTIR, CHNS elemental analysis, atomic absorption spectrophotometry and NMR. The effect of metal counter ion on the thermal and aggregation behaviour of CTAC was studied by TGA, conductivity and surface tension. The results reveal that metal incorporation decreases the CMC value and increases the thermal stability in the order Cu(II) > Co(II) > Ni(II) > Fe(II). The study of absorption and fluorescence spectra of fluorescein in pre and post micellar concentration of metallosurfactants was carried out. The binding constant and number of binding sites was evaluated by using Stern–Volmer equation. Fluorescence quantum yield (ΦF) of fluorescein (FL) was premeditated in prepared metallosurfactants (FeC I, CoC I, NiC I, CuC I). This single alkyl chain system was used to gain reliable insight into the contribution of metal towards the ΦF of FL. The analysis of results indicate that among all, copper surfactant exhibited high fluorescence quantum yield due to increase in the radiative decay of FL. Even as the concentration of metallosurfactant goes from pre-micellar to post-micellar, the ΦF of FL was increased.
Introduction
Surfactants are considered as great contributors towards colloidal chemistry with new approaches such as nanoelectronics,1 liquid crystals,2 mesoporous materials3 and drug delivery.4 The introduction of transition/lanthanide metals in surfactant molecules provides the opportunity for the study of innovative metallosurfactants with different functionalities5 that are unobtainable with conventional surfactants. The location of the metal also affects the surfactant applications. The metal may be concentrated in the polar head group,6 tail7 or as the counter ion8 of the surfactant. The incorporation of metals is particularly relevant due to the rich chemistry associated with multiple coordination numbers, geometrics and a number of redox states. A classic example is the ferrum laurate metallosurfactant with three alkyl chains that was obtained by reacting ferric chloride with sodium laurate. This metallosurfactant was further applied for the construction of reverse vesicles with different morphologies in different organic solvents.9In this study, we endeavour to study a metallosurfactant with metal as part of the counter ion. By changing the counter ion, various properties such as stability,10 critical micelle concentration11 and aggregation behaviour12 of the surfactant are affected. As a result, surfactants created with metals as part of the counter ion is a key step for diverse applications in the field of surfactant science. Brown et al.13 demonstrated a magnetic surfactant with a gadolinium counter ion. This magnetic surfactant exhibits low cytotoxicity and achieves rapid protein separation in the presence of a low magnetic field. Herein, our interest is directed towards the fabrication and application of new surfactants with different transition metals, specifically Fe2+, Co2+, Ni2+, and Cu2+ as part of the counter ion. Metals when present as part of the counter ion offer a number of advantages in catalysis,14 anticancer treatment by inhibiting DNA replication,15 drug delivery,16 interactions with proteins such as Bovine Serum Albumin (BSA)/Human Serum Albumin (HSA), magnetic resonance imaging17 and nanoparticles.18
Among their many applications, dye–surfactant systems underline remarkable achievements for industrial applications,19 chemical research20 and dye-separation processes.21 A study on the solubilization of a dye in micellar media has received great interest during the last few years due to the low solubility of dyes in water. A recent study by Kurniasih et al.22 focused on the solubilization of Nile red in aqueous surfactant and micellar solutions of hexadecyltrimethyl ammonium bromide, and their spectroscopic results revealed the absorption behaviour of Nile red at the micellar surface. The role played by metallosurfactants in the solubilization of dyes has not yet been explored much. Such systems differ from conventional surfactants in several aspects. In fact, the metal has a property to modify the physicochemical process of dyes by amplifying the incident light field that interacts with the d-electrons of the metal and increases the fluorescence quantum yield of the fluorophore.23 Hong et al. studied the metal enhanced fluorescence of surfactant-coated carbon nanotubes on nanostructured gold substrates. The surfactant coated carbon nanotubes showed an enhancement in fluorescence quantum yield due to the resonance coupling of the nanotubes emission to the dipolar plasmonic modes in the metal.24 Metals have also been used to determine the partition coefficient of dyes between the micellar and aqueous phase. The equilibrium constant for the metal–dye interaction has also been studied, which follows the same order as the Irving William series of stability of metal complexes.25 In fact, the presence of paramagnetic lanthanide ions increases the intersystem crossing of sensitizers, which is achieved by reducing the distance between the sensitizer and lanthanide ion by exploiting the carboxylate group of fluorescein.26
The solvatochromic probe, fluorescein (FL), is one of the most popular anionic dyes with expanding applications in chemistry and biology, which however suffers from poor solubility. FL derivatives have been used as chemosensors for Hg2+ and Ag2+ by Shen et al.,27 and for Au3+ by Kambam et al.,28 and their cytotoxicity assays exhibited their further use in human living cells for sensing. FL was also used for the detection of different aggregates of short-tailed and long-tailed cationic calix[4]arene in water by Cheipesh et al.29 The binding mechanism of FL with HSA had been investigated in micellar media using steady-state and time resolved spectroscopy. The binding mechanism indicates the fluorescence quenching of the tryptophan residue in protein, whereas the binding constant evaluates the moderate binding strength of the FL–HSA complex.30 In fact, the spectral properties, i.e. the ground and excited state of FL, are affected by surfactant monomers and micellar media.31
Therefore, the objective of the present paper is to synthesize surfactants with different transition metals as part of the counter ion in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. For this purpose hexadecyltrimethyl ammonium chloride (CTAC) is used as the precursor to prepare metallosurfactants with metal chlorides (Fe, Co, Ni and Cu) as part of their counter ion. All four complexes are characterized using FTIR, CHNS elemental analysis, atomic absorption spectrophotometry (AAS) and NMR. The thermal behaviour of the complexes are analysed by thermogravimetric analysis (TGA). To understand the effect of the metal on the thermal stability of the complexes, five non-isothermal methods are applied. Conductivity and surface tension methods are used to determine the aggregation behaviour of the metal complexes. This work further investigates the solubilization and fluorescence quantum yield of FL in micellar media of metallosurfactants. Spectroscopic analysis is used for the determination of binding sites and the location of the dye in the metallo-micelles. Investigation of the metal enhanced fluorescence quantum yield of dyes in micellar media in the present study will be beneficial in a variety of industrial, scientific and medical applications.
1 stoichiometry. For this purpose hexadecyltrimethyl ammonium chloride (CTAC) is used as the precursor to prepare metallosurfactants with metal chlorides (Fe, Co, Ni and Cu) as part of their counter ion. All four complexes are characterized using FTIR, CHNS elemental analysis, atomic absorption spectrophotometry (AAS) and NMR. The thermal behaviour of the complexes are analysed by thermogravimetric analysis (TGA). To understand the effect of the metal on the thermal stability of the complexes, five non-isothermal methods are applied. Conductivity and surface tension methods are used to determine the aggregation behaviour of the metal complexes. This work further investigates the solubilization and fluorescence quantum yield of FL in micellar media of metallosurfactants. Spectroscopic analysis is used for the determination of binding sites and the location of the dye in the metallo-micelles. Investigation of the metal enhanced fluorescence quantum yield of dyes in micellar media in the present study will be beneficial in a variety of industrial, scientific and medical applications.
Materials
Iron(II) chloride (FeCl2), cobalt(II) chloride (CoCl2), nickel(II) chloride hexahydrate (NiCl2·6H2O), copper(II) chloride dihydrate (CuCl2·2H2O) (purity ≥ 99.0%) and hexadecyltrimethyl ammonium chloride (CTAC) (purity ≥ 98%) were purchased from Sigma Aldrich. Fluorescein (purity ≥ 95%) and D2O were also received from Sigma Aldrich. NaH2PO4 and Na2HPO4 used to prepare phosphate buffer were of analytical grade. Ethanol (purity ≥ 99.9%) was purchased from Changshu Yangyuan Chemical, China. Deionised and purified water was used for conductivity, surface tension measurements and buffer preparation.Synthesis and characterization of metallosurfactants
Metallosurfactants containing a single alkyl chain with sixteen carbons (CTAC) and different metal ions (as the counter ion) were prepared via the ligand insertion method. In brief, 0.4 g metal chloride (Fe, Co, Ni, and Cu) and 1 g quaternary ammonium chloride were solubilised in 20 mL ethanol and heated to reflux for 2 h at 60 °C. Then, ethanol was evaporated using a rotary evaporator and the complexes were isolated as solid powders with different respective colours. Before analyses, they were recrystallized in methanol.The CHN content of the complexes (FeC I, CoC I, NiC I, and CuC I) were estimated using a Thermo Scientific (FLASH 2000) analyser and metal concentration was analysed on a Phoenix-986 atomic absorption spectrophotometer (AAS). Spectrometric methods were used for the characterization of the synthesized metallosurfactants. IR spectra (4000–400 cm−1) were obtained on a Perkin Elmer (RX1) spectrometer and FTIR spectra (50–400 cm−1) were obtained on a Perkin Elmer (Spectrum 400) spectrometer with 100 scans and a 4 cm−1 spectral resolution using KBr plates. Thermogravimetric analyses were conducted using an SDT-Q-600 (TA instruments) to calculate the changes in the physical and chemical properties of the formulated complexes as a function of temperature. Samples were heated at a rate of 10 °C min−1 in an aluminium crucible from room temperature to 1000 °C. For structural studies, XRD was carried out using a Panalytical's X'Pert Pro X-ray diffractometer operated with Cu-Kα radiation with a 2Q angle in the 5–80° range. The aggregation behaviour of the prepared metal complexes was evaluated by conductivity and tensiometric measurements performed using a Pico Lab India digital conductivity meter and Du Nouy Tensiometer (Kruss type 8451), respectively. Double distilled water with a conductivity of less than 5 μS cm−1 and surface tension of 71 mN m−1 at 25 °C was used for these experiments. The temperature was maintained by a thermostat (±0.01 °C) in the range of 25–40 °C. Two readings were obtained for each sample to determine any change with time and to obtain an average value.
For photophysical properties, the absorption and emission spectra of FL with the metal complexes were recorded on a Jasco V-530 (1 cm quartz cell) and Hitachi F-7000 photoluminescence spectrophotometer. FL and the samples were excited at 460 nm with emission spectra recorded in the range of 520–540 nm for the dye. The emission and excitation slit widths were kept at 10 nm and the scanning speed was 1200 nm min−1. The stock solution of metallosurfactant in the concentration range of 0.05 to 2.0 mM and dye of 0.05 mM were prepared in phosphate buffer.
Results and discussion
Structural behaviour
Structural confirmation of the prepared complexes is provided by elemental analyses and atomic absorption spectroscopy. The CHN data given in Table ES1† indicate that the calculated percentages of C, H, and N are in close agreement with the experimental values. AAS was also carried out to determine the metal content in the metallosurfactants (Table ES1†).The vibrational bands observed in FTIR spectra are used for the determination of the mode of coordination of the metal ion with the surfactant. The IR peaks at 1473 cm−1 and 960 cm−1 correspond to the C–H stretching frequency and –CH2 rocking, respectively. As expected, no changes were observed in the major vibrational peaks at around 2915.26 and 2848.41 cm−1 for the asymmetric and symmetric stretching vibration of the alkyl chain, respectively, in all four metal complexes of CTAC. The other major peak associated with the complexes is 1262 cm−1, which represents the N–CH3 symmetric stretching vibration. This peak displayed minor shifts, which suggest the occurrence of complexation between the metal ions and the chloride ion of CTAC (Fig. ES1†). Besides this, in Fig. 1, the band at 325 cm−1 corresponding to the terminal metal chloride in the metal salts is blue shifted in the metal–surfactant complexes, and a new peak appears at approximately 280 cm−1 due to the metal–chloride bridging in all the metal complexes after coordination (Table ES2†). The reason for this shifting may be an alteration in the electron cloud of chlorine upon coordination with the metal ions. In the 1H-NMR spectra of CTAC and the metal complexes in D2O, a number of peaks are observed at different δ values, which are related to the different proton signals given in Table ES3.† In the metal complexes, there was a slight shift in the peaks, as seen in Fig. ES2,† which is attributed to the coordination of the metal ion with CTAC. However, in the case of FeC I, border peaks are observed due to interference because of its high paramagnetic properties.
The thermal degradation of the FeC I, CoC I, NiC I and CuC I complexes with DTG are depicted in Fig. 2(a). As observed, the degradation of the FeC I complex occurs in two steps. The first step is assigned to the loss of the quaternary ammonium structure in the temperature range of 200–450 °C and next step is ascribed to the decomposition of the metal chloride above 450 °C.8 Similarly, all other complexes exhibit the same decomposition pattern as described above. We also conclude that observed and calculated mass loss percentages are in good agreement with each other for all the complexes. In addition, the kinetics of different reaction methods are applied for all four metal complexes. Kinetic parameters, such as activation energy and pre-exponential factor, were obtained by five non-isothermal methods, namely the Coats–Redfern (CR), Horowitz–Metzger (HM), Madhusudanan Krishnan–Ninan (MKN), Van Krevelen (VK) and Wanjun–Yuwen–Hen–Cunxin (WYHC) methods. These methods were assessed on the basis of α (degree of conversion), β (heating rate) and g(α) differential conversion functions. α and (1 − α)n were calculated from the TG curves, where n is the order of reaction.32 The activation energy (E) was calculated using the abovementioned methods and the respective graphs are provided in Fig. ES3;† the other parameters ΔG, ΔH and ΔS, were calculated using the CR method and are depicted in Table ES4.† The detailed equations and graphs are provided in the ESI.†
According to the calculated activation energy by the five abovementioned methods (Table 1), the Cu(II) complex possesses the highest activation energy among the four complexes. This means that Cu(II) is thermally more stable compared to the other three complexes, and stability follows the order Cu(II) > Co(II) > Ni(II) > Fe(II), which is same as that of the ionic radius size of the metal ions except for nickel. The same pattern of thermal stability was also obtained by Arshad et al.33 where a metal was complexed with the 1,2-diphenyl ligand. This is based on the fact that the smaller sizes of metal ions allow a closer approach to the ligand which leads to a higher metal–ligand bond energy (i.e. activation energy). However, in the case of the Ni complex, the TG curves indicate the presence of a small amount of water molecules in the complex that causes a disturbance in the molecular structure of the complex and this results in a decrease in the thermal stability of the Ni complex. As evident from the activation energy of the complexes, it is confirmed that each metal with a specific bond strength coordinates with the ligand and alters the thermal behaviour of the metal complexes. The negative entropy of the metal complexes (Table ES4†) also indicates that the formulated metal complexes are more ordered systems than their respective parent surfactant.
| Metal complex | Transition temp. (°C) | Mass loss% | E/kJ mol−1 | |||||
|---|---|---|---|---|---|---|---|---|
| Cal. | Obs. | CR | MKN | WYHC | VK | HM | ||
| FeC I | 273.42 | 71.71 | 73.73 | 21.49 | 21.67 | 21.73 | 23.69 | 26.03 |
| CoC I | 281.27 | 71.58 | 71.38 | 36.17 | 36.79 | 36.82 | 41.23 | 39.54 |
| NiC I | 295.29 | 71.26 | 74.13 | 27.96 | 28.10 | 28.15 | 32.50 | 32.75 |
| CuC I | 296.10 | 70.50 | 71.44 | 49.85 | 49.68 | 49.73 | 54.38 | 53.90 |
In X-ray diffraction, X-rays strike the electrons of atoms and produce diffracted waves that may be directed in a constructive or destructive way, which is determined by Bragg's law. In XRD, the solid powders of the metal surfactant complexes show a reasonable degree of crystallinity, as indicated in Fig. 2(b) except for the nickel complex. The intensity of the reflections at 2Q = 6.6 and 11.2° increases with a decrease in the radii of the metal ion coordinated with CTAC, except in the nickel complex due to more hydration of the cation, as is also evident from the TGA data.
Micellization
Micellization is a term used for the determination of the aggregation behaviour of surfactants. Plots of electrical conductivity and surface tension versus concentration for the aqueous solutions of metallosurfactants depict the critical micelle concentration (CMC) where micelles are formed (Fig. 3). The chloride ion of CTAC was exchanged with [MCl3]− in the metallosurfactants, where M = Fe, Co, Ni, and Cu. In fact, it is found that the metallosurfactants having a metallic counter ion with the metal ion present as a co-ion have lower CMC as compared to CTAC (with Cl− as the counter ion). This profound effect on CMC is often used to obtain information on the characteristic properties of the metal ion and its position in the metallosurfactant structure. In the hydrated metallosurfactant (Scheme 1), the Cl− counter ion is closer to the metal co-ion due to the +2 nuclear charge. When the metallosurfactant is dissolved in water, there is a change in colour due to the hydration of the metal ion present as a co-ion in the diffuse layer.34 This leads to an overall increase in the size of the counter ion layer, which reduces the cation–cation head group repulsion, and thus decreases the CMC value of the metallosurfactant (Table 2). | ||
| Fig. 3 (a) Variation of conductivity at different temperatures and (b) surface tension of the metal complexes at 25 °C with a variation in concentration. | ||
| Complex | Temp. (°C) | CMC (mM) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (kJ K−1 mol−1) | Γmax (μmol m−2) | Amin (nm)2 | |
|---|---|---|---|---|---|---|---|---|
| κ | γ | |||||||
| CTAC | 25 | 1.58 | 1.55 | −39.35 | −10.19 | 0.090 | 1.53 | 1.08 |
| FeC I | 25 | 1.03 | 1.00 | −30.56 | −8.03 | 0.075 | 0.91 | 1.81 |
| 30 | 1.11 | −34.20 | −9.20 | 0.082 | ||||
| 35 | 1.15 | −32.98 | −9.04 | 0.077 | ||||
| 40 | 1.20 | −31.86 | −8.91 | 0.073 | ||||
| CoC I | 25 | 1.10 | 0.99 | −30.68 | −5.82 | 0.083 | 0.87 | 1.90 |
| 30 | 1.15 | −30.93 | −5.99 | 0.082 | ||||
| 35 | 1.19 | −30.88 | −6.10 | 0.080 | ||||
| 40 | 1.22 | −33.37 | −6.72 | 0.085 | ||||
| NiC I | 25 | 1.15 | 0.96 | −29.11 | −2.57 | 0.089 | 0.49 | 3.38 |
| 30 | 1.17 | −30.64 | −2.76 | 0.092 | ||||
| 35 | 1.18 | −30.30 | −2.77 | 0.089 | ||||
| 40 | 1.21 | −31.77 | −2.96 | 0.092 | ||||
| CuC I | 25 | 1.12 | 1.07 | −27.93 | −4.18 | 0.079 | 0.38 | 4.31 |
| 30 | 1.18 | −29.44 | −4.49 | 0.082 | ||||
| 35 | 1.21 | −31.86 | −4.95 | 0.087 | ||||
| 40 | 1.29 | −31.45 | −4.98 | 0.084 | ||||
The temperature dependence of the CMC profiles of the different systems was also obtained. It is found that as the temperature of the system increases, there is a distortion of the structured water molecules due to the weaker hydration of the hydrophobic groups of the metallosurfactant and this impedes micellization.35 The other thermodynamic parameters of micellization were also calculated using the following equations, where ΔG°, ΔH° and ΔS° are the Gibbs free energy, change in enthalpy and change in entropy, respectively.
ΔG° = (2 − β)RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XCMC XCMC
| (1) |
ΔH° = −RT2(2 − β)dln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XCMC/dt XCMC/dt
| (2) |
| ΔS° = (ΔH° − ΔG°)/T | (3) |
| Complex | Kb (×103 M−1) | Ka (×103 M−1) | n | Concentration (mM) | ΦF |
|---|---|---|---|---|---|
| FeC I | 0.15 | 3.63 | 0.560 | 0.50 × 10−3 | 0.23 |
| 1.00 × 10−3 | 0.40 | ||||
| 1.50 × 10−3 | 0.60 | ||||
| CoC I | 0.62 | 2.52 | 0.525 | 0.50 × 10−3 | 0.19 |
| 1.00 × 10−3 | 0.36 | ||||
| 1.50 × 10−3 | 0.47 | ||||
| NiC I | 1.36 | 2.61 | 1.054 | 0.50 × 10−3 | 0.30 |
| 1.00 × 10−3 | 0.51 | ||||
| 1.50 × 10−3 | 0.48 | ||||
| CuC I | 1.37 | 2.50 | 0.894 | 0.50 × 10−3 | 0.24 |
| 1.00 × 10−3 | 0.68 | ||||
| 1.50 × 10−3 | 0.95 |
As observed from ESI Fig. ES4,† entropy dominance changed to enthalpy dominance with an increase in temperature. The entropy contribution to free energy at low temperature is also due to the devastation of water molecules around the hydrocarbon chain. The compensation phenomenon acknowledges the participation of water in micellization through the structural properties of water or interactions with the solute. This phenomenon gives an estimation of the solute–solvent interaction through TC and solute–solute interaction by σ. These values are derived from the plot of ΔH° vs. ΔS°, where TC is obtained from the slope and σ from the intercept, as listed in Table ES5.† The lower value of TC in the metal complexes is an indication of prominent solute–solute interactions which help in the solvation part of micellization.
The surface tension of the metal complexes as a function of concentration (at 25 °C) was measured to obtain their CMC values, as shown in Fig. 3(b). The results indicate that surface tension decreases with an increase in the concentration of the metal complexes and then reaches a plateau zone, which is symbolic of micelle formation. By inspecting the data, it is deduced that metallosurfactants have a higher efficiency in decreasing the surface tension of solvent. In addition, surface tension is related to the maximum surface concentration and minimum surface area of metallosurfactant molecules adsorbed at the interface, which are calculated from the Gibbs adsorption equation.
 | (4) |
 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) is the surface pressure, R is the universal gas constant and T is the absolute temperature. For the metallosurfactants, Γmax was less and Amin was higher than CTAC. During the micellization of the metallosurfactants, the increased number of co-ions near the counter ions leads to weak interactions between the quaternary ammonium groups and bound counter ions, which reduce the close packing of surfactant molecules at the interface.36
C) is the surface pressure, R is the universal gas constant and T is the absolute temperature. For the metallosurfactants, Γmax was less and Amin was higher than CTAC. During the micellization of the metallosurfactants, the increased number of co-ions near the counter ions leads to weak interactions between the quaternary ammonium groups and bound counter ions, which reduce the close packing of surfactant molecules at the interface.36
Interaction with xanthene dye
The sensitizing effect of the metal based surfactants at their pre and post micellar concentration on the spectroscopic behavior of xanthene dye, i.e. fluorescein, was studied by absorption and fluorescence spectroscopy. FL is a highly fluorescent molecule and its high fluorescence intensity is used in several applications such as labelling proteins in fluorescence microscopy.37 However, quenching and low solubility in aqueous solution38 reduce its fluorescence quantum yield. In the fluorescence spectrum of FL, the peak observed at 490 nm in aqueous solution is shifted at 500 nm in the presence of the metallosurfactants at their pre-micellar concentrations. These results support the formation of ion-pair aggregation between the dye and metallosurfactant. As observed in Fig. 4(a), the extent of the enhancement of the absorption maxima in the order of Fe(II) > Co(II) > Ni(II) > Cu(II) is probably the result of the lower d-orbital electron number.39 This allows more dye molecules to be adsorbed on the surface of the surfactant. At a certain point corresponding to the CMC of the metallosurfactants, the solubilization tendency of FL molecules into micelles reaches a limiting value and dimer–monomer equilibrium of dye molecules reorients towards monomer to decrease the absorbance.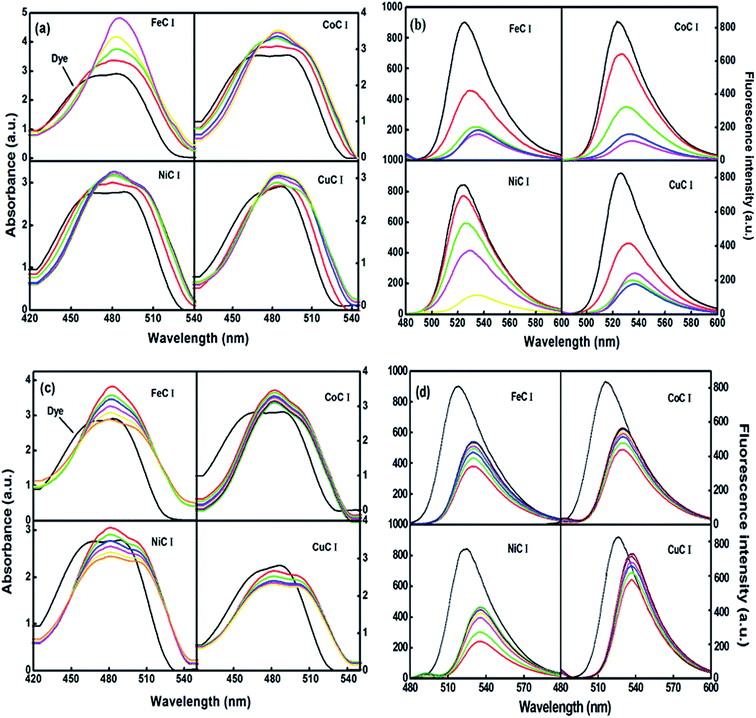 | ||
| Fig. 4 Absorption and emission spectra of FL with the metal complexes at pre-micellar concentrations (a and b) and post micellar concentrations (c and d) respectively. | ||
 | ||
| Fig. 5 Binding plots of FL with the metal complexes through UV (a–d) and fluorescence (e–h) studies. | ||
Furthermore, the peak in the fluorescence spectrum of FL at 525 nm is due to π–π* transition, and it is noted that its intensity decreases with a red shift in the presence of a pre-micellar concentration of metallosurfactants (Fig. 4(b)), which depends on the polarity and size of the transition metal. As the size of the metal ion decreases, the effective nuclear charge increases, which enhances the polarity of the head group of the metallosurfactant and leads to more interaction with the dye in its π* (excited) state than in the π (ground) state.40 This decreases the ΔE (energy gap), which corresponds to a high bathochromic shift in the Cu(II) metallosurfactant as compared to the other three metallosurfactants. At post micellar concentrations, the dye is expected to be completely solubilized in the hydrophobic core of micelles and the results indicate an increase in the fluorescence intensity of FL (Fig. 4(d)). This may be due to the lower intramolecular motion of the dye because of its interaction with the hydrophobic core of the micelles, which reduces the non-radiative emission in the excited state.8
Binding constants were determined using absorption and emission spectra of FL in the presence of the metallosurfactants, and these values are reported in Table 3. The binding constant was calculated from the UV spectra for the ground state interaction of FL with the metallosurfactants by fitting data in the Benesi–Hildebrand equation (Fig. 5(a)–(d)).
| Ao/A − Ao = (εG/εH–G − εG) + (εG/εH–G − εG)(1/Kb[Lt]) | (6) |
The description of the binding mechanism was classified by applying a modified Stern–Volmer equation, where a plot of log(fo − f/f) versus log[Q] gave a straight line whose intercept and slope defined the binding constant (Ka) and number of binding sites (n) respectively as shown in Fig. 5(e)–(h).
 | (7) |
The value of n, as given in Table 3, indicates that FL can interact with the metallomicelles through only one site, which may be the fragment of the carboxylic group, compared to CTAC micelles in which FL is bound at two binding sites.8 This is due to the presence of metal co-ions with the counter ion. It is inferred from the abovementioned observations that the lower the number of d-electrons in the orbital of the metal ion, the more interactive the metallomicelles with FL.
By comparing the reference absorption and emission spectra, the fluorescence quantum yield (ΦF) of FL in micellar solutions of the different transition metallosurfactants was measured. For this purpose, FL in 0.01 M NaOH solution was chosen as the standard reference and the influence of the presence of metal ions on the ΦF of FL dye was elucidated.41 The ΦF of FL at different concentrations of metallosurfactants was calculated using following equation, and the data is provided in Table 3.
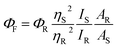 | (8) |
Conclusion
Four new transition metal–surfactant complexes in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry were synthesized with a hexadecyltrimethyl ammonium head group and MCl3 counter ion. These metal complexes were structurally characterized by different techniques such as CHNS, FTIR, AAS, NMR and XRD. Investigation of the metal effect on the aggregation behaviour of the single chain complex reveals a decrease in the CMC of the surfactant due to the presence of the divalent metal co-ion. The temperature dependent CMC profile of the complexes show that there is a small effect of temperature on the CMC of the complexes due to the small difference in the hydration power of the co-ions. Furthermore, the spectroscopic analysis reveal the formation of ion-pair aggregates between FL and the metallosurfactants at pre CMC and complete solubilization of FL in metallomicelles post CMC. The results of this study indicate that metal-enriched surfactant complexes enhance the fluorescence quantum yield of fluorescein. Significantly, this metal effect opens new perspectives for the understanding of interfacial behaviour and application of fluorescein in biological systems as an excellent fluorescent probe.
1 stoichiometry were synthesized with a hexadecyltrimethyl ammonium head group and MCl3 counter ion. These metal complexes were structurally characterized by different techniques such as CHNS, FTIR, AAS, NMR and XRD. Investigation of the metal effect on the aggregation behaviour of the single chain complex reveals a decrease in the CMC of the surfactant due to the presence of the divalent metal co-ion. The temperature dependent CMC profile of the complexes show that there is a small effect of temperature on the CMC of the complexes due to the small difference in the hydration power of the co-ions. Furthermore, the spectroscopic analysis reveal the formation of ion-pair aggregates between FL and the metallosurfactants at pre CMC and complete solubilization of FL in metallomicelles post CMC. The results of this study indicate that metal-enriched surfactant complexes enhance the fluorescence quantum yield of fluorescein. Significantly, this metal effect opens new perspectives for the understanding of interfacial behaviour and application of fluorescein in biological systems as an excellent fluorescent probe.
Acknowledgements
Preeti Garg is grateful to UGC-India for Junior research fellowship. Gurpreet Kaur is thankful to DST for Inspire Faculty award (IFA-12-CH-41). Ganga Ram Chaudhary would like to acknowledge the support of UGC, India under INDO-US 21st century knowledge Initiative project [F. No. 194-2/2016(IC)]. Authors are also thankful to DST for PURSE grant II.References
- S. Kim, Y. Jang, K. Y. Yoon and J. Park, Surface engineered gold nanoparticles through highly stable metal–surfactant complexes, J. Colloid Interface Sci., 2016, 464, 110–116 CrossRef CAS PubMed.
- A. Baruah, A. K. Pathak and K. Ojha, Study on the Thermal Stability of Viscoelastic Surfactant-Based Fluids Bearing Lamellar Structures, Ind. Eng. Chem. Res., 2016, 54, 7640–7649 CrossRef.
- C. He, X. Liu, J. Shi, C. Ma, H. Pan and G. Li, Anionic starch-induced Cu-based composite with flake-like mesostructure for gas-phase propanal efficient removal, J. Colloid Interface Sci., 2015, 454, 216–225 CrossRef CAS PubMed.
- S. Ghosh, A. Ray, N. Pramanik and B. Ambade, Can a catanionic surfactant mixture act as a drug delivery vehicle, C. R. Chim., 2016, 1–4 Search PubMed.
- G. Kaur, S. Kumar, N. Dilbaghi, B. Kaur, R. Kant, S. K. Guru, S. Bhushan and S. Jagland, Evaluation of bishexadecyltrimethyl ammonium palladium tetrachloride based dual functional colloidal carrier as an antimicrobial and anticancer agent, Dalton Trans., 2016, 6582–6591 RSC.
- G. R. Chaudhary, P. Singh, G. Kaur, S. K. Mehta, S. Kumar and N. Dilbaghi, Multifaceted approach for the fabrication of metallomicelles and metallic nanoparticles using solvophobic bisdodecylamine palladium(II) chloride as precursor, Inorg. Chem., 2015, 54, 9002–9012 CrossRef CAS PubMed.
- E. Parera, M. M. Garcia, R. Pons, F. Comelles, J. Suades and R. B. Rodriguez, Supramolecular arrangement of molybdenum carbonyl metallosurfactants with CO-releasing properties, Organometallics, 2016, 35, 484–493 CrossRef CAS.
- G. Kaur, P. Garg and G. R. Chaudhary, Role of manganese-based surfactant towards solubilization and photophysical properties of fluorescein, RSC Adv., 2016, 6, 7066–7077 RSC.
- R. Dong and J. Hao, Reverse vesicles of ferrum laurate metallosurfactant in non-aqueous solution dried to produce solid shells, ChemPhysChem, 2012, 13, 3794–3797 CrossRef CAS PubMed.
- G. N. Smith, P. Brown, C. James, R. Kemp, A. M. Khan, T. S. Plivelic, S. E. Rogers and J. Easto, The effects of counterion exchange on charge stabilization for anionic surfactants in nonpolar solvents, J. Colloid Interface Sci., 2016, 465, 316–322 CrossRef CAS PubMed.
- G. N. Smith, P. Brown, C. James, S. E. Rogers and J. Easto, The effect of solvent and counterion variation on inverse micelle CMCs in hydrocarbon solvents, Colloids Surf., A, 2016, 494, 194–200 CrossRef CAS.
- M. J. Pottage, T. L. Greaves, C. J. Garvey and R. F. Tabor, The effects of alkylammonium counterions on the aggregation of fluorinated surfactants and surfactant ionic liquids, J. Colloid Interface Sci., 2016, 475, 72–81 CrossRef CAS PubMed.
- P. Brown, L. Bromberg, M. I. R. Hermida, M. Wasbrough, T. A. Hatton and C. A. Lorenzo, Magnetic surfactants and polymers with gadolinium counterions for protein separations, Langmuir, 2016, 32, 699–705 CrossRef CAS PubMed.
- T. Yagyu, M. Tonami, K. Tsuchimoto, C. Takahashi and K. Jitsukawa, Preparation of palladium(II) complexes with long alkyl chain ligand incorporated in micelle, Inorg. Chim. Acta, 2012, 392, 428–432 CrossRef CAS.
- A. M. Badawi, M. A. S. Mohamed, M. Z. Mohamed and M. M. Khowdairy, Surface and antitumor activity of some novel metal-based cationic surfactants, J. Cancer Res. Ther., 2007, 4, 198–206 Search PubMed.
- M. Benko, N. Varga, D. Sebok, G. Bohus, A. Juhasz and I. Dekany, Bovine serum albumin sodium alkyl sulfates bioconjugates as drug delivery systems, Colloids Surf., B, 2015, 130, 126–132 CrossRef CAS PubMed.
- Y. Chen, Q. Zhu, Y. Tian, W. Tang, F. Pan, R. Xiong, Y. Yuan and A. Hu, Supramolecular aggregates from polyacrylates and Gd(III)-containing cationic surfactants as high-relaxivity MRI contrast agents, Polym. Chem., 2015, 6, 1521–1526 RSC.
- R. Kaur and S. K. Mehta, Self-aggregating metal surfactant complexes: precursors for nanostructures, Coord. Chem. Rev., 2014, 262, 37–54 CrossRef CAS.
- S. Baliarsingh, J. Jena, T. Das and N. B. Das, Role of cationic and anionic surfactants in textile dyeing with natural dyes extracted from waste plant materials and their potential antimicrobial properties, Ind. Crops Prod., 2013, 50, 618–624 CrossRef CAS.
- L. Das, S. Chatterjee, D. B. Naik and S. Adhikari, Role of surfactant derived intermediates in the efficacy and mechanism for radiation chemical degradation of a hydrophobic azo dye, 1-phenylazo-2-naphthol, J. Hazard. Mater., 2015, 298, 19–27 CrossRef CAS PubMed.
- C. Muthukumaran, V. M. Sivakumar and M. Thirumarimurugan, Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent, J. Taiwan Inst. Chem. Eng., 2016, 63, 354–362 CrossRef CAS.
- I. N. Kurniasih, H. Liang, P. C. Mohr, G. Khot, J. P. Rabe and A. Mohr, Nile red dye in aqueous surfactant and micellar solution, Langmuir, 2015, 31, 2639–2648 CrossRef CAS PubMed.
- C. D. Geddes, I. Gryczynski, J. Malicka, Z. Gryczynski and J. R. Lakowicz, Metal-enhanced fluorescence: potential applications in HTS, Comb. Chem. High Throughput Screening, 2003, 6, 109–117 CrossRef CAS PubMed.
- G. Hong, S. M. Tabakman, K. Welsher, H. Wang, X. Wang and H. Dai, Metal-enhanced fluorescence of carbon nanotubes, J. Am. Chem. Soc., 2010, 132, 15920–15923 CrossRef CAS PubMed.
- J. K. Basu, M. Shannigrahi, N. Ray and S. Bagchi, UV-vis spectroscopic study of interaction of metal ions with the ET(30) dye involving micellar media, Spectrochim. Acta, Part A, 2005, 61, 2539–2542 CrossRef PubMed.
- G. A. Hebbink, L. Grave, L. A. Woldering, D. N. Reinhoudt and F. C. J. M. Veggel, Unexpected sensitization efficiency of the near-infrared Nd3+, Er3+ and Yb3+ emission by fluorescein compared to eosin and erythrosin, J. Phys. Chem. A, 2003, 107, 2483–2491 CrossRef CAS.
- W. Shen, L. Wang, M. Wu and X. Bao, A fluorescein derivative FLTC as a chemosensor for Hg2+ and Ag+ and its application in living-cell imaging, Inorg. Chem. Commun., 2016, 70, 107–110 CrossRef CAS.
- S. Kambam, B. Wang, F. Wang, Y. Wang, H. Chen, J. Yin and X. Chen, A highly sensitive and selective fluorescein-based fluorescence probe for Au3+ and its application in living cell imaging, Sens. Actuators, B, 2015, 209, 1005–1010 CrossRef CAS.
- T. A. Cheipesh, E. S. Zagorulko, N. O. M. Petrossyan, R. V. Rodik and V. I. Kalchenko, The difference between the aggregates of short-tailed and long-tailed cationic calix[4]arene in water as detected using fluorescein dyes, J. Mol. Liq., 2014, 193, 232–238 CrossRef CAS.
- O. K. Abou-Zied and S. A. J. Sulaiman, Site-specific recognition of fluorescein by human serum albumin: a steady-state and time-resolved spectroscopic study, Dyes Pigm., 2014, 110, 89–96 CrossRef CAS.
- S. Biswas, S. C. Bhattacharya, P. K. Sen and S. P. Moulik, Absorption and emission spectroscopic studies of fluorescein dye in alkanol, micellar and microemulsion media, J. Photochem. Photobiol., A, 1999, 123, 121–128 CrossRef CAS.
- G. Kaur, G. Karir and S. K. Mehta, Studies on thermogravimetric analysis and solvophobic interactions of micellization of Pd(II) complex in non-aqueous solvents, Colloids Surf., A, 2013, 434, 25–34 CrossRef CAS.
- M. Arshad, S. Rehman, S. A. Khan, K. Masud, N. Arshad and A. Ghani, A thermal analysis study of 1,2-dipiperidinoethane complexes of cobalt, nickel, copper, zinc and cadmium by TG-DTG-DTA techniques, Thermochim. Acta, 2000, 364, 143–149 CrossRef CAS.
- G. Kaur, S. Kumar, N. Dilbaghi, G. Bhanjana, S. K. Guru, S. Bhushan, S. Jaglan, P. Hassane and V. K. Aswal, Hybrid surfactants decorated with copper ion: aggregation behavior, antimicrobial activity and anti-proliferative effect, Phys. Chem. Chem. Phys., 2016, 18, 23961–23970 RSC.
- O. S. Esan, O. M. Olubunmi, A. C. Olumuyiwa and O. Olarenwaju, Effects of temperature and tetramethylammonium bromide salt on the micellization of cetyltrimethyl ammonium bromide in aqueous medium: a conductometric studies, Int. J. Thermodyn., 2015, 18, 246–252 CrossRef.
- S. M. Shaban, I. Aiad, M. M. El-Sukkary, E. A. Soliman and M. Y. El-Awady, Synthesis, surface, thermodynamic properties and biological activity of dimethylamino propylamine surfactants, J. Ind. Eng. Chem., 2014, 20, 4194–4201 CrossRef CAS.
- M. Tian, X. L. Wu, B. Zhang, J. L. Li and Z. Shi, Synthesis of chlorinated fluoresceins for labeling proteins, Bioorg. Med. Chem. Lett., 2008, 18, 1977–1979 CrossRef CAS PubMed.
- M. Arık, N. Celebi and Y. Onganer, Fluorescence quenching of fluorescein with molecular oxygen in solution, J. Photochem. Photobiol., A, 2005, 170, 105–111 CrossRef.
- G. Kurtoglu, B. Avar, H. Zengin, M. Kose, K. Sayin and M. Kurtoglu, A novel azo azomethine based fluorescent dye and its Co(II) and Cu(II) metal chelates, J. Mol. Liq., 2014, 200, 105–114 CrossRef CAS.
- S. K. Ghosh, M. Ali and H. Chatterjee, Studies on the interaction of fluorescein isothiocyanate and its sugar analogues with cetyltrimethylammonium bromide, Chem. Phys. Lett., 2013, 561, 147–152 CrossRef.
- J. Hadjianestis and J. Nikokavouras, Luminal chemiluminescence in micellar media II: energy transfer to fluorescein, J. Photochem. Photobiol., A, 1993, 69, 337–343 CrossRef.
- Z. Jin, X. Zhang, G. Lu and S. Li, Improved quantum yield for photocatalytic hydrogen generation under visible light irradiation over eosin sensitized TiO2—investigation of different noble metal loading, J. Mol. Catal. A: Chem., 2006, 259, 275–280 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21811c |
| This journal is © The Royal Society of Chemistry 2016 |



