Fast enhancement on bondability of wheat straw surface for bio-composites manufacture via dielectric barrier discharge plasma
W. M. Chenab,
Y. C. Xua,
S. K. Shia,
N. Thiphuonga,
M. Z. Chena and
X. Y. Zhou*ab
aCollege of Materials Science and Engineering, Nanjing Forestry University, No. 159 Longpan Road, Nanjing 210037, China
bNanjing Suman Plasma Technology Co., Ltd, Enterprise of Graduate Research Station of Jiangsu Province, No. 3 Youyihe Road, Nanjing 210001, China. E-mail: zhouxiaoyan@njfu.edu.cn; Fax: +86 25 8542 8517; Tel: +86 25 8542 8506
First published on 28th October 2016
Abstract
Waxy layer in outer surface of wheat straw (WS) has greatly limited its application in bio-composites manufacture owing to the poor bondability. Dielectric barrier discharge (DBD) plasma using glycidyl methacrylate (GMA) was applied in outer surface of WS in order to enhance the bondability for bio-composites manufacture. The chemical properties, morphology, and wettability of WS surface after GMA plasma treatment were investigated by attenuated total reflection infrared spectroscopy (ATR-IR), X-ray photoelectron spectroscopy (XPS), Boehm titration, atomic force microscopy (AFM), scanning electron microscopy (SEM), and optical contact angle measurement (OCA). Moreover, the two most commonly used adhesives, namely urea-formaldehyde resin (UF) and phenol formaldehyde resin (PF), were applied to evaluate the surface bondability of WS-based composites. The obtained results showed that numerous polar functional groups (C–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and COOH) were incorporated into WS surface. Surface oxidation and etching effects by GMA plasma treatment result in the appearance of numerous spots and the increase in surface roughness. Moreover, the dramatic increase in surface free energy and attenuation coefficients (K) were observed, indicating the improved wettability. The increased roughness, improved wettability, and enhanced polarity favor the dispersion and permeation process of adhesives and the formation of nail-like adhesives. These facts synergistically improve the bondability of wheat straw surface, demonstrating the increased shearing strength from 0.068 MPa to 1.423 MPa when using PF as adhesive.
O, and COOH) were incorporated into WS surface. Surface oxidation and etching effects by GMA plasma treatment result in the appearance of numerous spots and the increase in surface roughness. Moreover, the dramatic increase in surface free energy and attenuation coefficients (K) were observed, indicating the improved wettability. The increased roughness, improved wettability, and enhanced polarity favor the dispersion and permeation process of adhesives and the formation of nail-like adhesives. These facts synergistically improve the bondability of wheat straw surface, demonstrating the increased shearing strength from 0.068 MPa to 1.423 MPa when using PF as adhesive.
1. Introduction
In comparison to woody biomass, crop materials such as wheat straw have lower content of cellulose and lignin, but higher content of pentosan. Wheat straw also shows high ash content in which silica accounts for more than 90%.1,2 These facts have greatly limited its application in bio-composites and panel manufacture. Moreover, the higher pH and acid buffering capacity of aqueous extracts in wheat straw increase the curing time of urea-formaldehyde resin and decline the bondability.1,3 The poor wettability of wheat straw surface makes the adhesive difficult to spread and permeate owing to the presence of non-polar extracts and waxy layers.4 In general, wheat straw surface is chemically incompatible with the two commonly used adhesive, namely urea-formaldehyde resin (UF) and phenol formaldehyde resin (PF), which greatly decreases its bondability and thus limits its application in bio-composites and panel manufacture.5Many modification methods were applied in wheat straw surface to enhance its bondability. Han et al. reported that steam explosion treatment and extraction on wheat straw surface lead to the improvement on acidity and wettability. Moreover, this method causes a dramatic decrease in the content of silicon, which significantly enhances the bondability between straw particles and water-soluble adhesive binders.1 Han has also found that the wax-like substances and non-polar extracts can be effectively extracted from wheat straw surface using ethanol/benzene as organic solvents, which leads to significant improvement on its bondability.6 However, those modification methods are energy- and time-consuming. A more effective modification method for high bondability of wheat straw surface is attractive in order to simultaneously reduce the cost of bio-composites production and keep the low-value added wheat straw more competitive.
In recent years, plasma treatment has been considered as a promising modification technique on materials surface due to the advantages of solvent-free, well-controlled, simple operation procedure, non-pollution, and short processing period.7 Moreover, plasma treatment can simply alter the feeding gas type (O2, CO2, air, NH3, and N2, etc.) to produce different chemically active species on materials surface without changing its bulk properties significantly.8,9 Zhou et al. reported that composite of poplar fibres and oxygen plasma-treated enzymatic hydrolysis lignin can be prepared via self-gluing without any adhesive. The oxygen-containing functional groups were incorporated and phenoxy radicals were generated on lignin surface during radio frequency (RF) plasma treatment leading to significant improvement on self-bondability.10 Tang has found that the RF plasma treatment can significantly improve the wettability of poplar veneer surface.11 Yang has researched the dynamic wettability of RF plasma treated wheat straw surface using three types of adhesives, namely urea-formaldehyde (UF), phenol-formaldehyde (PF), and methylene diphenyl diisocyanate (MDI) resins.12 However, dielectric barrier discharge (DBD) plasma can produce more reactive oxidizing species and uniform discharge in comparison to RF plasma. In addition, less mass loss can be achieved when it is applied in materials surface. Chen has used the DBD plasma treatment on woody poplar veneer surface and investigated the effects of processing rate on poplar veneer surface.13 To the best of our knowledge, there is rarely literature related to DBD plasma treatment on crops material, particularly wheat straw. The enhancement mechanism for bondability via DBD plasma treatment remains unknown. In comparison to O2, H2O, and air, glycidyl methacrylate (GMA) contains more polar functional groups, which were easily excited by DBD plasma treatment. We assumed that GMA has better modification effects on wheat straw surface for the enhancement on its bondability.
In the present study, DBD plasma using GMA was applied in WS surface in order to enhance its bondability for bio-composites manufacture. The chemical properties, morphology, and wettability of WS surface after GMA plasma treatment were investigated by a series of characterization methods. Moreover, the two most commonly used adhesives, namely urea-formaldehyde resin (UF) and phenol formaldehyde resin (PF), were applied, sequentially, to evaluate the surface bondability of WS-based composites.
2. Material and methods
2.1 Materials
WS used in this study was purchased from a local factory in Nanjing city. It was first cleaned with distilled water to remove impurities and cut into pieces with the size of 50 mm × 5 mm, then dried at 103 °C in a oven for 6 hours.UF was self-prepared in a laboratory of Nanjing Forestry University. Basic properties were presented as follow: solid content (2 h, 120 °C), 55.10%; pH (20 °C), 7.40; viscosity (20°), 128.00 mPa s; density, 1.12 g cm−3; surface tension, 84.86 mJ m−2. PF was purchased from Taier Chemical Industry Co. Ltd. Its basic properties were shown as follow: solid content (2 h, 120 °C), 52.00%; pH (20 °C), 9.80; viscosity (20 °C), 270.00 mPa s; density, 1.21 g cm−3; surface tension, 93.38 mJ m−2. Both the adhesives were applied to evaluate the surface wettability of WS and shear strength of WS-based bio-composites.
2.2 DBD plasma modification
Fig. 1 shows the schematic of the experimental setup for GMA DBD plasma treatment. The reactor in DBD plasma system is composed of a circular parallel-plate (diameter, 50 mm; barrier thickness; 3.0 mm, distance between the two barriers, 5.0 mm). Tempered glass and stainless steel were used for the barrier materials and electrodes, respectively. After a series of preliminary experiments, a discharge power of 40 W, a reactor chamber pressure of 20 KPa, and a treating time of 120 s were determined in this study, which achieved the highest shear strength of WS-based bio-composites. WS pieces were placed in the reactor chamber with its outer face upward. The GMA was heated up to 95 °C by a water bath and the reactor chamber pressure was pumped to 20 KPa by a vacuum pump in order to make the reactor chamber filled with GMA steam. Large numbers of polar reactive species (atoms and ions) derived from GMA are excited by DBD plasma via applying an AC power.2.3 Characterization methods
Chemical structure of WS was revealed by attenuated total reflection infrared spectroscopy (ATR-IR). All samples were scanned and recorded in the wavenumber range of 400–4000 cm−1 with a resolution of 4 cm−1.The surface compositions of atoms and functional groups of WC were revealed using X-ray photoelectron spectroscopy (XPS, AXIS Ultra DLD). Low-resolution spectra with the binding energy ranging from 0 eV to 1200 eV were recorded using survey scanning mode. The high-resolution spectra of C1s (277–296 eV) were also recorded (pass energy, 10 eV; non-monochromatic Mg Kα, hγ = 1253.7 eV; Al Kα X-radiations, hγ = 1486.7 eV). The spectra of C1s were deconvoluted into 4 Gaussian peaks corresponding to 4 carbon-related components using XPSPEAK Software (version 4.1).
Surface acidic functional groups of WS were investigated using Boehm's titration. A piece of WS was added into 0.05 L of one of the three reaction bases (0.05 M NaOH, NaHCO3, and Na2CO3) and into 0.05 L of 0.05 M HCl. A magnetic stirrer was applied to agitate the obtained suspensions for 24 h which were subsequently subjected to filtration. 10 mL filtrate was pipetted and titrated using 0.05 M HCl to determine the content of acidic functional groups.
Surface morphology of WS was investigated using scanning electron microscopy (SEM) and atomic force microscopy (AFM). For the characterization with SEM, WS was cut into pieces with a square size of 5 mm × 5 mm and dried in a vacuum oven at 60 °C for 6 h, then subjected to gold-coating process by a PECS coating machine in order to reduce the charging effects. For the characterization of AFM, the WS was cut into a size of 40 mm × 2 mm using Franklin maceration and placed into boiling water. The water started to be poured out when the WS pieces sank to the bottom of the test tube. A solution consisting of hydrogen peroxide and glacial acetic acid with the volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was added into the test tube and heated until the white color in WS was achieved. Then, the white floc of WS was washed using distilled water to neutral conditions. WS fibers were obtained until the white floc was shaken to be fully dispersed. Drops consist of distilled water and WS fibers were dripped onto a mica sheet in order to eliminate moisture, then dried at 40 °C in a vacuum oven for 6 h. WS fibers were subjected to DBD plasma treatment. Finally, both of the plasma treated and untreated WS fiber loaded mica sheets were, respectively, attached to the observation stage of AFM (XE-100) for imaging. The software of XEI was applied to obtain the 2D section lines of WS fiber surface and calculate the roughness parameters of Ra and Rb.
1 was added into the test tube and heated until the white color in WS was achieved. Then, the white floc of WS was washed using distilled water to neutral conditions. WS fibers were obtained until the white floc was shaken to be fully dispersed. Drops consist of distilled water and WS fibers were dripped onto a mica sheet in order to eliminate moisture, then dried at 40 °C in a vacuum oven for 6 h. WS fibers were subjected to DBD plasma treatment. Finally, both of the plasma treated and untreated WS fiber loaded mica sheets were, respectively, attached to the observation stage of AFM (XE-100) for imaging. The software of XEI was applied to obtain the 2D section lines of WS fiber surface and calculate the roughness parameters of Ra and Rb.
An optical contact angle measuring apparatus (theta) was used to determine the contact angles and evaluate the wettability of WS surface. Water, diiodomethane, UF, and PF (volume at 20 °C, 4 μL) were, respectively, used as testing drops dispersed on the outer surface of WS. A camera was connected to a computer to capture the images of the dispersing process for every 50 milliseconds. The initial contact angle was determined when the drop just reached the WS surface, whereas the equilibrium contact angle was determined when the drop shape was stable. The contact angles of distilled water and diiodomethane were used to calculate the total surface free energy by applying the Owens–Wendt method in which distilled water was used to determine the surface free energy of polar components, whereas diiodomethane was used for surface free energy of non-polar components. The contact angles of UF and PF were applied to evaluate the surface bondability of WS-based composites. A attenuation coefficient (K) was defined as the rate of contact angles varied with time prolonged and determined using the equation as shown below.14
2.4 DBD plasma modification
WS pieces were coated with UF and PF (including 1% NH4Cl as curing agent), respectively, with a coating size of 15 mm × 5 mm and assembled as shown in Fig. 2. Then, those assembled materials were subjected to a manual press machine for preparing WS-based bio-composites via hot pressing. The experimental conditions were presented as follows: adhesive content in single side, 200 g cm−3; hot pressing pressure, 1.5 MPa; hot pressing temperature, 110 °C; hot pressing time, 90 s. The bondability of WS-based bio-composites was evaluated by the shear strengths, which were determined based on the methods established by our research groups.12 | ||
| Fig. 2 WS-based bio-composites types (OP, outer surface of wheat straw treated by plasma; OU, outer surface of untreated wheat straw; IU, inner surface of untreated wheat straw). | ||
3. Results and discussion
3.1 Chemical analysis
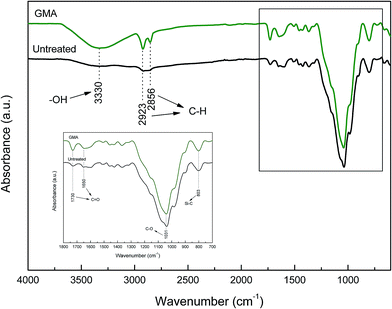
Fig. 3 shows the ATR-IR spectra of untreated wheat straw and plasma treated one. According to the previous studies,15,16 the adsorption peak at 3330 cm−1 is attributed to –OH. The bands at 1730 cm−1 and 1650 cm−1 represent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Moreover, C–O was recorded at the absorption peak of 1031 cm−1. Both of these peaks exhibit significantly higher intensity after plasma treatment, which implies that a large number of oxygen-containing free radicals and functional groups were generated (e.g., –OH and ·OH) and successfully incorporated into wheat straw surface during plasma treatment. C–H bands were recorded at 2923 cm−1 and 2856 cm−1, showing higher intensity after plasma treatment. This fact was mainly related to the generation of ·CH3 and ·CH2· from GMA and subsequent incorporation into the wheat straw surface. In addition, the adsorption band at 803 cm−1 indicates the presence of C–Si demonstrating higher intensity after plasma treatment. This result is attributed to the etching effect on surface waxy layer leading to the exposure of Si from the endodermis.
O. Moreover, C–O was recorded at the absorption peak of 1031 cm−1. Both of these peaks exhibit significantly higher intensity after plasma treatment, which implies that a large number of oxygen-containing free radicals and functional groups were generated (e.g., –OH and ·OH) and successfully incorporated into wheat straw surface during plasma treatment. C–H bands were recorded at 2923 cm−1 and 2856 cm−1, showing higher intensity after plasma treatment. This fact was mainly related to the generation of ·CH3 and ·CH2· from GMA and subsequent incorporation into the wheat straw surface. In addition, the adsorption band at 803 cm−1 indicates the presence of C–Si demonstrating higher intensity after plasma treatment. This result is attributed to the etching effect on surface waxy layer leading to the exposure of Si from the endodermis.
In order to reveal the composition of surface atomic elements and chemical functional groups, X-ray photoelectron spectroscopy (XPS) was performed on the surface of untreated wheat straw and plasma treated one.17 The results were presented in Fig. 4 and Table 1. It can be concluded that oxygen content was dramatically increased after plasma treatment, showing the successful oxygen incorporation onto the wheat straw surface. Moreover, according to previous studies,18,19 the C1s spectra of the wheat straw surface can be deconvoluted into 4 Gaussian peaks corresponding to 4 carbon-related chemical functional groups (C–C/C–H, 284.6 eV; C–OH, 286.5 eV; C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 287.8 eV; COOH, 288.8 eV). GMA plasma treatment leads to the significant increase in the content of oxygen-containing groups (C–OH, C
O, 287.8 eV; COOH, 288.8 eV). GMA plasma treatment leads to the significant increase in the content of oxygen-containing groups (C–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, COOH), particularly C–OH, indicating the enhancement on polarity of wheat straw surface, whereas an evident decrease in the content of C–C was observed after plasma treatment. Ion bombardment caused by plasma treatment on wheat straw surface leads to the rupture of C–C and generation of active sites and free radicals which subsequently reacted with the carbonyl, carboxyl, and ester groups excited from GMA. Those facts are responsible for the enhancement of the polarity of wheat straw surface. Table 2 shows the surface acidic functional groups of untreated WS and plasma treated one. It can be concluded that GMA plasma treatment leads to an increase in the content of all oxygenated functional groups, which is in good agreement with the XPS results.
O, COOH), particularly C–OH, indicating the enhancement on polarity of wheat straw surface, whereas an evident decrease in the content of C–C was observed after plasma treatment. Ion bombardment caused by plasma treatment on wheat straw surface leads to the rupture of C–C and generation of active sites and free radicals which subsequently reacted with the carbonyl, carboxyl, and ester groups excited from GMA. Those facts are responsible for the enhancement of the polarity of wheat straw surface. Table 2 shows the surface acidic functional groups of untreated WS and plasma treated one. It can be concluded that GMA plasma treatment leads to an increase in the content of all oxygenated functional groups, which is in good agreement with the XPS results.
 | ||
| Fig. 4 XPS spectra of survey scanning and high resolution for untreated wheat straw and plasma treated one. | ||
| C1s (%) | Atomic components (%) | Surface free energy (mJ m−2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| –C–C– | –C–OH | –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
–O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
C | O | Si | T | P | N | |
| a T, surface total free energy; P, surface free energy of polar components; N, surface free energy of non-polar components. | ||||||||||
| Untreated | 84.5 | 10.2 | 3.7 | 1.6 | 90.9 | 7.3 | 0.7 | 34.7 | 7.3 | 27.4 |
| GMA | 31.8 | 41.4 | 12.6 | 14.2 | 35.3 | 48.4 | 15.2 | 64.6 | 32.5 | 32.1 |
| Carboxylic (mmol g−1) | Lactonic (mmol g−1) | Phenolic (mmol g−1) | Acidic (mmol g−1) | |
|---|---|---|---|---|
| Untreated | 0.06 | 0.07 | 0.10 | 0.23 |
| GMA | 0.23 | 0.12 | 0.15 | 0.50 |
3.2 Morphological analysis
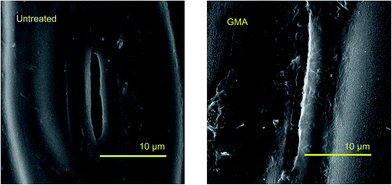
AFM and SEM were applied to observe the surface morphology of untreated wheat straw and plasma treated one and the results were presented in Fig. 5 and 6. A relatively smooth surface in untreated wheat straw was observed owing to its inherent molecular structure and distribution, whereas large numbers of spots appeared after plasma treatment leading to the increase in surface roughness. This result may be attributed to the surface etching effects and surface oxidation by plasma treatment. Moreover, AFM was also used to evaluate the surface roughness by defining the 2D section lines and calculating the roughness parameters of Ra and Rb. The results were presented in Fig. 7. Only one peak (maximum height of 203.2 nm) was observed in untreated wheat straw, whereas several peaks and troughs (maximum height of 681.8 nm, minimum height of 27.7 nm) appeared continuously in the plasma treated one. In addition, Ra and Rb were increased by 498.1% and 435.5%, respectively. Those results indicate that surface roughness was significantly increased by GMA plasma treatment. The aromatic or aliphatic polymers in WS surface were degraded by the charged particles during DBD plasma treatment, which results in the etching effects altering the microscopic morphology and increasing the surface roughness.20 It should be noted that roughness of wheat straw favors the formation of nail-like adhesive leading to the enhancement on bondability of bio-composite.21 | ||
| Fig. 7 2D section lines for the surface of untreated wheat straw and treated one (Ra, arithmetic average roughness; Rb, root mean square roughness). | ||
3.3 Surface wettability analysis
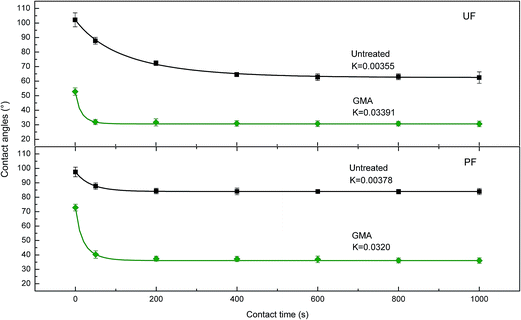
Two most commonly used adhesives, namely UF and PF, were used for contact angle measurements, respectively. The results were shown in Fig. 8. A dramatic decrease was observed in the values of both initial contact angle and equilibrium contact angle after plasma treatment. Particularly, initial contact angle of wheat straw surface treated by GMA plasma showed a decrease by 48.32% and reached 52.8° using UF as droplet. In addition, plasma treatment leads to a significantly decrease by 57.12% in the value of equilibrium contact angle and reached 36.1° using PF as droplet. Moreover, all attenuation coefficient (K) shows good correlation coefficient (>0.95). The attenuation coefficients (K) using both UF and PF as droplet were nearly increased by tenfold after GMA plasma treatment. Those facts demonstrated good wettability of wheat straw surface, which were attributed to the large numbers of polar chemical groups grafted into wheat straw surface during plasma treatment. It should be noted that good wettability favors the adhesive fast spreading and permeating into the materials surface resulting in the improvement on bondability of bio-composites.22,23 Surface free energy of untreated wheat straw and the plasma treated one is shown in Table 1. It can be concluded that total surface free energy was increased by 86.2% after plasma treatment in which 72.6% of the increase was contributed by polar components. This fact was related to the successful incorporation of polar chemical groups (e.g., carbonyl, carboxyl, and ester groups), which agreed well with XPS and FTIR analysis.3.4 Bondability analysis and its enhancement mechanism
The shear strengths of WS-based bio-composites were measured and applied to evaluate the bondability. Table 3 shows the bondability of untreated wheat straw and plasma treated one. It can be observed that the samples using PF adhesive demonstrate higher shear strengths than that using UF adhesive. This fact is related to the inherent high viscosity of PF and network cross-linked structure formed after solidification. The composite of OU–OU exhibits the lowest shear strengths (0.034 MPa) owing to the poor bondability of wax layer in outer surface of wheat straw. Composite of OP–OU shows a dramatic increase in the value of shear strengths after GMA plasma treatment. Moreover, highest shear strength was achieved in the composite of OP–OP using PF adhesive and reached 1.423 MPa. These results directly prove the strong enhancement effect by GMA plasma on the bondability of wheat straw surface. The enhancement mechanism was proposed in Fig. 9. During GMA plasma treatment, degradation and devolatilization of WC surface occurred, leading to the formation of surface roughness and the generation of activated sites, which are originated from the rupture of C–C bond (confirmed by XPS results). Moreover, GMA molecules are exited and decomposed into a series of products with radical sites. These products subsequently interacted with the free radicals of activated sites and non-activated sites of WS surface, resulting in the successful incorporation of polar chemical functional groups. In summary, the increased roughness, improved wettability, and enhanced polarity favor the dispersion and permeation process of adhesive and the formation of nail-like adhesive. These facts synergistically improve the bondability of wheat straw surface.| OU–OU | OP–OU | OU–IU | OP–IU | OP–OP | |
|---|---|---|---|---|---|
| a OU, untreated outer surface of wheat straw; OP, plasma treated one; IU, untreated inner surface of wheat straw; ‘A–B’ in this table represents the bio-composite whose adhesion interface was formed between A and B. | |||||
| UF (MPa) | 0.034 ± 0.003 | 0.342 ± 0.067 | 0.092 ± 0.018 | 0.726 ± 0.127 | 1.049 ± 0.191 |
| PF (MPa) | 0.068 ± 0.013 | 0.493 ± 0.085 | 0.123 ± 0.024 | 0.741 ± 0.111 | 1.423 ± 0.214 |
4. Conclusion
The active sites and free radicals generated by plasma treatment were reacted with the excited carbonyl, carboxyl, and ester groups from GMA leading to the successful incorporation of polar functional groups (C–OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and COOH) into wheat straw surface. Surface oxidation and etching effect by GMA plasma treatment results in the appearance of numerous spots and the increase in surface roughness. Moreover, a dramatical increase in surface free energy and attenuation coefficients (K) was observed after plasma treatment, indicating the improved wettability. The increased roughness, improved wettability, and enhanced polarity favor the dispersion and permeation process of adhesive and the formation of nail-like adhesive. Those facts synergistically improve the bondability of wheat straw surface, demonstrating the increased shearing strength of bio-composite from 0.068 MPa to 1.423 MPa when using PF as adhesive.
O, and COOH) into wheat straw surface. Surface oxidation and etching effect by GMA plasma treatment results in the appearance of numerous spots and the increase in surface roughness. Moreover, a dramatical increase in surface free energy and attenuation coefficients (K) was observed after plasma treatment, indicating the improved wettability. The increased roughness, improved wettability, and enhanced polarity favor the dispersion and permeation process of adhesive and the formation of nail-like adhesive. Those facts synergistically improve the bondability of wheat straw surface, demonstrating the increased shearing strength of bio-composite from 0.068 MPa to 1.423 MPa when using PF as adhesive.
Acknowledgements
The authors are grateful for the support by projects from the National Natural Science Foundation of China (Grant No. 31270606 and Grant No. 31400515), the Natural Science Foundation of Jiangsu Province (Grant No. BK20161524), the Doctorate Fellows Foundation of Nanjing Forestry University, the Jiangsu Province Ordinary University Students' Scientific Research Innovation Project (Grant No. KYZZ16_0320), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Also this paper was sponsored by Qing Lan Project.References
- G. P. Han, J. Deng, S. Y. Zhang, P. Bicho and Q. L. Wu, Ind. Crops Prod., 2010, 31, 28 CrossRef CAS.
- I. Kellersztein and A. Dotan, Polym. Compos., 2016, 37, 2133 CrossRef CAS.
- M. Singh, A. Kaushik and D. Ahuja, Carbohydr. Polym., 2016, 150, 48 CrossRef CAS PubMed.
- A. Parenti, E. Muguerza, A. R. Iroz, A. Omarini, E. Conde, M. Alfaro, R. Castanera, F. Santoyo, L. Ramirez and A. G. Pisabarro, Bioresour. Technol., 2013, 133, 142 CrossRef CAS PubMed.
- W. L. Chen, G. P. Han, A. R. Iroz and D. Feng, BioResources, 2013, 8, 4497 Search PubMed.
- G. Han, K. Umemura, S. Kawai and H. Kajita, J. Wood Sci., 1999, 45, 299 CrossRef CAS.
- H. C. Huang, D. Q. Ye and B. C. Huang, Surf. Coat. Technol., 2007, 201, 9533 CrossRef CAS.
- G. Lota, J. Tyczkowski, R. Kapica, K. Lota and E. Frackowiak, J. Power Sources, 2010, 195, 7535 CrossRef CAS.
- J. Y. Zhou, Z. W. Wang, R. Zuo, Y. Zhou, X. M. Cao and K. Cheng, Asia-Pac. J. Chem. Eng., 2012, 7, 245 CrossRef.
- X. Y. Zhou, F. Zheng, C. L. Lv, L. J. Tang, K. C. Wei, X. Y. Liu, G. B. Du, Q. Yong and G. Xue, Composites, Part B, 2013, 53, 369 CrossRef CAS.
- L. J. Tang, R. Zhang, X. Y. Zhou, M. Z. Chen, X. Y. Yang, P. Zhou and Z. Chen, BioResources, 2012, 7, 3327 CrossRef.
- X. H. Yang, R. Zhang, L. J. Tang, M. Z. Chen, Y. Li and X. Y. Zhou, BioResources, 2014, 9, 1578 Search PubMed.
- M. Z. Chen, R. Zhangm, L. J. Tang, X. Y. Zhou, Y. Li and X. H. Yang, BioResources, 2016, 11, 1571 Search PubMed.
- S. Q. Shi and D. J. Gardner, Wood Fiber Sci., 2001, 33, 58 CAS.
- Z. Kadar, N. Schultz-Jensen, J. S. Jensen, M. A. T. Hansen, F. Leipold and A. B. Bjerre, Biomass Bioenergy, 2015, 81, 26 CrossRef CAS.
- S. R. A. Collins, N. Wellner, I. M. Bordonado, A. L. Harper, C. N. Miller, I. Bancroft and K. W. Waldron, Biotechnol. Biofuels, 2014, 7, 121 CrossRef PubMed.
- H. Sun, W. C. Hockaday, C. A. Masiello and K. Zygourakis, Ind. Eng. Chem. Res., 2012, 51, 3587 CrossRef CAS.
- W. M. Chen, S. K. Shi, T. Nguyen, M. Z. Chen and X. Y. Zhou, BioResources, 2016, 11, 3923 CAS.
- R. Azargohar, S. Nanda, J. A. Kozinski, A. K. Dalai and R. Sutarto, Fuel, 2014, 125, 90 CrossRef CAS.
- A. Jamali and P. D. Evans, Wood Sci. Technol., 2011, 45, 169 CrossRef CAS.
- H. Moghadamzadeh, H. Rahimi, M. Asadollahzadeh and A. R. Hemmati, Int. J. Adhes. Adhes., 2011, 31, 816 CrossRef CAS.
- S. H. Ghaffar and M. Fan, Biomass Bioenergy, 2015, 83, 373 CrossRef CAS.
- Z. Liu, F. Wang and X. Wang, Wood Fiber Sci., 2004, 36, 239 CAS.
| This journal is © The Royal Society of Chemistry 2016 |