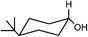
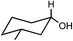
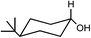
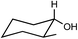
Preparation and application of surface activated Si-MCM-41 and SBA-16 as reusable supports for reduction of cyclic ketones with preferential stereoselectivity†
Haribandhu Chaudhuri,
Subhajit Dash and
Ashis Sarkar*
Organic Materials Research Laboratory, Department of Applied Chemistry, Indian School of Mines, Dhanbad, Jharkhand-826004, India. E-mail: a_sarkar_99@yahoo.com; Fax: +91 326 2307772; Tel: +91 9430335255
First published on 13th October 2016
Abstract
An efficient and benign procedure for the reduction of a few cyclic ketones adsorbed on the activated surface of calcined Si-MCM-41 and calcined SBA-16 using NaBH4 as the reducing agent with ethanol as the medium resulted in the formation of two epimeric alcohols. After synthesis, these calcined materials were treated with concentrated HCl to activate surface silanol groups. Various instrumental techniques like FTIR, XRD, N2 sorption isotherms, FESEM, TEM and XPS were carried out to examine the pre and post activated surface of the used supports. Five cyclic ketones were reduced. Reduction of 4-tert-butylcyclohexanone yielded only trans-4-tert-butylcyclohexanol. Moreover, exclusive formation of cis-3-methylcyclohexanol (equatorial –OH) was also observed. This work offers several advantages such as a simple operational procedure, short reaction time, and high yield of the product, along with maintaining the materials' diversity. This is due to the presence of the activated silanol groups of these materials, which cause nucleophilic activation of the carbonyl group of ketones leading to faster reaction rates. Beside this, these supports can be regenerated well from the reaction mixture using a calcination treatment followed by concentrated HCl, and reused several times without causing any serious malformation in the activated surfaces. Finally, this work opens up a new direction of research for the fabrication of solid reusable supports in the reduction of cyclic ketones.
1. Introduction
To be applicable as an excellent catalyst support, a material should have a number of minimal requirements, such as a remarkable stability under the working conditions, a fairly low-cost, a simple, user friendly synthetic method and unambiguous characteristics to evaluate the quality of the final synthesised material.1–6 Moreover, other requirements include suitability of the pores for the reagents along with controllable micro- and mesoporosity. The micro- and mesoporosity can greatly elevate the activity and selectivity of the catalyst. In this context, mesoporous silica materials have received a huge recognition due to their excellent surface area and pore volume as well as nano sized pores (mesopores). Specially, both Si-MCM-41 and SBA-16 have hexagonally ordered pores throughout the surface of these materials. Those surface properties make them useful as catalyst support.7–18 The amorphous nature of the silica walls most often results in poorer catalytic performance. So, it is necessary to prepare uniformly distributed and preferably isolated active sites on the mesoporous silica surface, which is crucial for high catalytic activity and selectivity.19 The catalytic application of Si-MCM-41 has received much attention for the past few years and the counterpart, SBA-16, has not yet been studied vigorously due to difficulties encountered during the synthesis procedure. But, the problem is now overcome and we have carried out a series of stereochemical investigations on both Si-MCM-41 and SBA-16 to make a new vista of research in the direction of stereochemistry and to check whether these materials are suitable as solid supports for stereochemical investigations.20,21 Previous workers have reported stereoselective reduction of some cyclic and bicyclic ketones with lithium trialkylaluminium hydride and lithium diisobutyl-tert-butylaluminium hydride.22,23 But, from the view point of stable isomer formation and % of isolated yield, these results were not very much exciting.So, from the understanding of that kind of problems, we introduce activated Si-MCM-41 and SBA-16 as supports for reduction of some cyclic ketones with preferential stereoselectivity using NaBH4 as reducing agent. We explored the possibility of using active mesoporous silicas as adsorbent for ketones towards effective nucleophilic activation in the reduction with sodium borohydride (NaBH4).24,25 The purpose was twofold (a) increase the rate of reduction (b) improvement of stereoselectivity in the reduction of some cyclic ketones. As a consequence, some used ketones were fully converted to stabler alcohols with greater chemical yields in short time, compared to that reported earlier.23 Our initial effort to reduce cyclic ketones with low carbon fly ash as adsorbent and NaBH4 as reducing agent was not very encouraging.26,27 Reduction of the cyclic ketones namely 4-tert-butylcyclohexanone, 3-methylcyclohexanone, 2-methylcyclohexanone, 3,3,5-trimethylcyclohexanone, and 4-methylcyclohexanone were accompanied by similar stereochemical results as obtained in the reduction of those ketones under homogeneous condition (NaBH4 in ethanol). There was neither improvement in the rates of the reductions nor in chemical yields. This problem has now been overcome by activation of surface silanol groups of these materials using concentrated HCl, a report is being made herewith.
2. Experimental section
2.1. Materials and reagents
Tetraethoxysilane (TEOS, ≥ 99%), CTAB ((C16H33)N(CH3)3Br, MW = 364.45), Pluronic F127 (EO106PO70EO106, MW = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600), chloroform-d (CDCl3), and tetramethylsilane (TMS) were purchased from Sigma Aldrich. All used ketones were obtained from Alfa Aesar. Triethanolamine (TEA, N(CH2CH2OH)3, ≥ 98%), hydrochloric acid (HCl, ∼ 37%), sodium borohydride (NaBH4), sodium sulphate (Na2SO4), potassium bromide (KBr), diethyl ether and ethanol (EtOH, ≥ 99%) were procured from Merck India. The pre-coated aluminium plates (Merck) were used for thin layer chromatography (TLC) and spots were taken under UV light. In column chromatography, we used silica gel (60–120 mesh). Deionised water was used without further purification.
600), chloroform-d (CDCl3), and tetramethylsilane (TMS) were purchased from Sigma Aldrich. All used ketones were obtained from Alfa Aesar. Triethanolamine (TEA, N(CH2CH2OH)3, ≥ 98%), hydrochloric acid (HCl, ∼ 37%), sodium borohydride (NaBH4), sodium sulphate (Na2SO4), potassium bromide (KBr), diethyl ether and ethanol (EtOH, ≥ 99%) were procured from Merck India. The pre-coated aluminium plates (Merck) were used for thin layer chromatography (TLC) and spots were taken under UV light. In column chromatography, we used silica gel (60–120 mesh). Deionised water was used without further purification.
2.2. Syntheses
Si-MCM-41 material was synthesised by a method where TEOS was added into aqueous medium containing CTAB, ethanol, and TEA. The following process was reported previously:28 2.56 mL of water (0.36 mol), 4.56 mL of ethanol (0.015 mol), 4.16 g of a 25 wt% CTAB solution (0.786 mmol), and 0.07 mL of TEA (0.19 mmol) were combined and stirred in an oil bath at 60 °C for 45 min. Then, 2.92 mL of TEOS (3.25 mmol) was added dropwise into the mixture and stirred for 2 h. Then, the white solution was cooled to room temperature and the solid product (yield ∼ 98%) was filtered, washed with deionised water and distilled ethanol. After that, the product was dried under vacuum at 70 °C for 12 h. Finally, the material was calcined at 550 °C for 6 h at a heating rate at 15 °C min−1.SBA-16 was prepared using a method comparable to that reported earlier.1 Pluronic F127 (4.0 g) was dissolved in 81 mL of 2 M HCl and 30 mL of deionised water with stirring for 2 h at room temperature. Then, 9.04 mL of TEOS was added to that homogeneous solution and stirred for 20 h at room temperature. The mixture was then aged at 80 °C for 24 h under invariable conditions. Thereafter, the solid product (yield ∼ 98%) was filtered, washed with deionised water, distilled ethanol and air-dried overnight. Calcination was carried out by slowly increasing the temperature to 500 °C at a heating rate of 20 °C min−1 and heating further at a constant temperature for 6 h.
After that, the virgin materials were refluxed in concentrated HCl for 12 h to activate the surface silanol groups. Thereafter, the materials were made acid free by washing with distilled water and dried by heating at 110 °C for 24 h.
2.3. General procedure for reduction of ketones
In a typical reduction procedure, different amount of ketones like 2 mmol, 5 mmol, and 10 mmol (respectively) were added to 1 g of acid treated calcined solid in presence of ether in different round bottom flask (RB) and stirred for 12 h each. After ensuring that the ketones have been thoroughly adsorbed on the surface of the activated solid, NaBH4 (2 mmol, 5 mmol, and 10 mmol, respectively) in 5 mL ethanol was added to the mixture and refluxed with stirring for certain time (Tables 2 and 3). Those reactions were monitored using TLC and 1H nuclear magnetic resonance (NMR) spectroscopy. After the reaction was complete, the ethanolic layer was separated and the residue extracted with 10 mL of ethanol. The combined ethanolic extract after drying over anhydrous Na2SO4 (2 g) was evaporated to obtain the corresponding alcohols. After reaction, the synthesised catalysts were collected in distilled water and separated using calcination at 500 °C followed by treatment with concentrated HCl under reflux condition and reused for the next reaction. All reduced products were characterized by TLC and 1H NMR.2.4. Characterisations
Fourier transformed infrared (FTIR) spectroscopic analyses were recorded using FTIR spectrometer (Model IR-Perkin Elmer, Spectrum 2000) using KBr pellet method. X-ray diffractograms (XRD) patterns were collected using Thermal ARL X-ray diffractometer associated with CuKα radiation source along with a graphite monochromator. N2 sorption measurements were done at 77 K with Nova 3200e (Quantachrome, USA). The morphologies of the acid treated calcined Si-MCM-41 and SBA-16 were determined using field emission scanning electron microscopy (FESEM, Supra 55, Zeiss, Germany) and transmission electron microscopy (TEM, JEM-2100, JEOL, Japan). Surface chemical state with elemental composition analysis was carried out using X-ray photoelectron spectroscopic (XPS) instrument (PHI 1600 ESCA system, Perkin Elmer). 1H NMR spectra were taken in Bruker advanced 300 MHz and 400 MHz spectrometers fitted with 5 mm broadband inverse probe using CDCl3 as solvent and TMS as internal standard. δ values are reported in parts per million (ppm) and coupling constants (J) in hertz (Hz).3. Results and discussion
3.1. Characterisations of activated materials
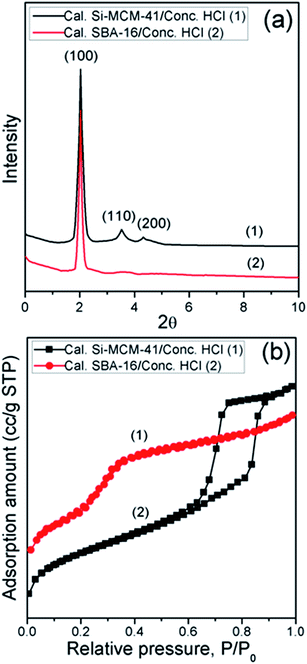
FTIR spectra of calcined materials and concentrated HCl treated materials are presented in Fig. 1a. The main characteristic peaks of calcined materials are: broad bands near 3462 cm−1 and 3468 cm−1 which are due to silanol –OH stretching vibrational mode. The corresponding bands due to bending vibrational mode are observed at 1639 cm−1 and 1637 cm−1 29,30 respectively. Asymmetric stretching vibrational band near 1087 cm−1 and 1085 cm−1 are due to the presence of Si–O–Si linkage in those calcined materials. The bands at 983 cm−1 and 986 cm−1 are characteristic one for the mesoporous materials31 and may be linked to Si–OH stretching vibration. Symmetric stretching bands of Si–O–Si near 803 cm−1 and 809 cm−1 are also observed. After activation of surface silanol groups using concentrated HCl, the main characteristic peaks that appear are: broad band near 3459 cm−1 and 3466 cm−1 which are due to silanol –OH stretching vibrational mode. The corresponding band due to bending vibrational modes are observed at 1638 cm−1 and 1641 cm−1 respectively.29 Asymmetric stretching vibrational bands near 1086 cm−1 and 1089 cm−1 are due to the presence of Si–O–Si linkage on those materials. The bands at 981 cm−1 and 982 cm−1 are important peaks for the mesoporous materials29 and may be linked to Si–OH stretching vibrations. Symmetric stretching band of Si–O–Si near 806 cm−1 and 807 cm−1 are also observed. So, there are no significant changes observed for concentrated HCl treated calcined materials. Only the peak intensities of –OH groups appeared much sharper due to the existence of activated silanol groups on the surface of those materials.XRD patterns of calcined materials and concentrated HCl treated materials are summarised in Fig. 1b. The obtained calcined Si-MCM-41 material exhibits some prominent peaks which are assigned to (100), (110), (200), and (210). These peaks are indicative of typical ordered mesoporous structures with hexagonal arrays.32 There are no any significant changes observed for concentrated HCl treated calcined Si-MCM-41 material. Only the assigned peak intensities decreased a little. On the other hand, the synthesized calcined SBA-16 exhibits typical low-angle diffraction patterns of mesoporous structures. These prominent peaks are assigned to (100), (110), and (200) which are typical of ordered mesoporous structures with hexagonal arrays. There are no significant changes observed for concentrated HCl treated calcined SBA-16 material. Only the (100) peak intensity decreased a little.
N2 sorption isotherms for the calcined materials and concentrated HCl treated calcined materials were carried out (Fig. 1c). Specific surface area, average pore diameter, and total pore volume data are given in Table 1. Brunauer–Emmett–Teller (BET) surface area for SBA-16 is 652 m2 g−1 having an average pore diameter of 5.71 nm with a total pore volume of 0.91 cm3 g−1. The increase of nitrogen uptake at P/P0 > 0.95 is a characteristic feature of this type of ordered mesoporous silicas and it is attributed to the presence of high textural porosity.33 As a result, calcined SBA-16 material possesses very high framework with average pore diameter and total pore volume. Concentrated HCl treated calcined SBA-16 possesses greater surface area (734 m2 g−1), average pore diameter (5.94 nm), and total pore volume (0.93 cm3 g−1) due to the presence of activated surface silanol groups on the material compared to the calcined material. Whereas, concentrated HCl treated calcined Si-MCM-41 possesses greater surface area (1265 m2 g−1), average pore diameter (3.67 nm), and total pore volume (1.05 cm3 g−1) compared to the calcined Si-MCM-41 material having BET surface area of 1202 m2 g−1 having an average pore diameter of 3.61 nm with a total pore volume of 1.02 cm3 g−1. This is due to the fact that after treatment of concentrated HCl, specific surface area, average pore diameter along with total pore volume increases thus retaining the surface phenomenon of these materials same as before.
| Materials | BET surface area (m2 g−1) | Average pore diameter (nm) | Total pore volume (cm3 g−1) |
|---|---|---|---|
| Cal. Si-MCM-41/Conc. HCl | 1256 | 3.67 | 1.05 |
| Cal. Si-MCM-41 | 1202 | 3.61 | 1.02 |
| Cal. SBA-16 | 652 | 5.71 | 0.91 |
| Cal. SBA-16/Conc. HCl | 734 | 5.94 | 0.93 |
Both FESEM and TEM are important tools for characterising the surface morphology of the material. It is useful for determining the particle shape, porosity and approximate size distribution of the material. Both FESEM and TEM were performed for concentrated HCl treated materials (Fig. 2). FESEM images reveal that fine particles have been formed (Fig. 2a and b). The hexagonal symmetries of the concentrated HCl treated materials were confirmed by TEM images (Fig. 2c and d) and the observation of particle shape (Fig. 2c) and ordered lattice array (Fig. 2d) suggests that the pore structure and size are well formed for these materials.
 | ||
| Fig. 2 (a) FESEM and (c) TEM spectra of concentrated HCl treated Si-MCM-41, respectively. (b) FESEM and (d) TEM spectra of concentrated HCl treated SBA-16, respectively. | ||
To study the chemical state, both calcined materials and acid treated calcined materials were analysed by the XPS technique (Fig. 3). According to the survey scan, only silicon and oxygen exist on the surface of pre and post acid treated materials. In order to examine the composition of these used materials, the core level Si(2p) peaks of XPS spectra have been studied. A perusal of Fig. 3(c and d) reveals that each Si(2p) peak consists of two Si(2p3/2) and Si(2p1/2) peaks due to the spin orbital coupling. The binding energy of Si(2p3/2) peaks in Fig. 3(c and d) was determined to be about 103.1 eV (Fig. 3c) and 102.9 eV (Fig. 3d) for pre and post acid treated materials, which are consistent with the binding energy for formation of siloxane moieties.34 Fig. 3(e and f) shows the deconvoluted O(1s) of pre and post acid treated mesoporous silicas. These peaks have been deconvoluted into two parts, in which binding energy of the first part is assigned to OH− and +OH2 groups, respectively and the second part corresponds to the oxygen bound in the H2O molecule. Furthermore, a perusal of Fig. 3e and f reveals that the presence of H2O molecules and OH−/+OH2 molar ratio is greater in acid treated calcined materials compared to the calcined materials.35 Therefore, based on the XPS analysis, it can be assumed that activation of mesoporous silica surfaces have been successfully done using acid treatment.
In this context, refluxing the calcined Si-MCM-41 and SBA-16 materials with conc. HCl yields activated surface silanol groups onto these materials in which –OH groups are protonated to produce +OH2, which is bigger in size compared to –OH group. Beside this, being a neutral molecule, it is a good leaving group. Moreover, because of the presence of –+OH2 groups, the Si atoms to which it is attached becomes more electron deficient. Thus, some small changes have been observed for acid treated calcined solids and the above discussions depict that the surfaces of these calcined materials have been activated successfully.
3.2. Stereochemical studies
Both acid treated calcined Si-MCM-41 and SBA-16 materials were used as adsorbents for five ketones, namely 4-tert-butylcyclohexanone, 2-methylcyclohexanone, 3-methylcyclohexanone, 4-methylcyclohexanone, 3,3,5-trimethylcyclohexanone and reduced with NaBH4. Those five ketones were chosen as the intention was to check whether there was any change in stereochemical outcome of the reduction using present systems. Moreover, their rates of reduction were points of interest.The obtained product, which should be a mixture of cis and trans-cyclohexanol, should give a complicated 1H NMR spectrum. However, we can easily use the spectrum to analyze the ratio of cis to trans isomers in our product because the methine hydrogen, the hydrogen on the carbon bearing the hydroxyl group, is shifted downfield of the other signals. Considering a mixture of cis and trans-4-tert-butylcyclohexanol, there is a signal at about δ = 3.54 ppm for the methine hydrogen of the trans isomer (ESI†) and a signal at about δ = 4.02 ppm for the methine hydrogen of the cis isomer (ESI†). The integration values for these two signals correspond to the relative amounts of the two isomers. The methine hydrogen of the trans isomer is axially placed whereas the methine hydrogen of the cis isomer is equatorially placed. Studies of many different substituted cyclohexanones have shown that axial hydrogen appear at about 0.5 ppm higher field than the corresponding equatorial hydrogen when cis and trans isomers are compared. Using this information we can analyse that the 4-tert-butylcyclohexanol formed consists only of trans-isomer and not the cis-isomer and reduction product of 3-methylcyclohexanone gives only of cis-isomer and not the trans-isomer. In this context, 1H NMR (CDCl3, 300 and 400 MHz, ppm) of 4-tert-butylcyclohexanol (δ 3.54, C–H axial proton), 2-methylcyclohexanol (δ 3.11, C–H axial proton and δ 3.76, C–H equatorial proton), 3-methylcyclohexanol (δ 3.58, C–H axial proton), 4-methylcyclohexanol (δ 3.54, C–H axial proton and δ 3.92, C–H equatorial proton), and 3,3,5-trimethylcyclohexanol (δ 3.76, C–H axial proton and δ 4.16, C–H equatorial proton) have been determined.26,27 All the 1H NMR spectra have been given in the (ESI†).
Tables 2 and 3 represent that the chemical yields (%) and time of completion (in h) for the reduction of ketones using acid treated calcined Si-MCM-41 and SBA-16 materials as supports and NaBH4 as reducing agent. A perusal of Tables 2 and 3 reveal that the overall chemical yields of various alcohols are very high and the time required for complete reductions are short. For complete reduction of 3,3,5-trimethylcyclohexanone, the reduction time was much longer as compared to other used ketones. 3,3,5-Trimethylcyclohexanone is a hindered ketone and the approach by the reducing species namely H− from either side is rather difficult. A perusal of Tables 4 and 5 reveals that the stereochemical outcomes of the reductions in case of 4-tert-butylcyclohexanone and 3-methylcyclohexanone are commendable. In both the cases, exclusive formation of thermodynamically stabler epimer takes place (in which the –OH groups are equatorially disposed). In case of other ketones like 2-methylcyclohexanone, the formation of stabler alcohols (–OH equatorial) are not exclusive (Tables 4 and 5). However, there is a greater preponderance of the stabler alcohol (–OH equatorial). In case of 3,3,5-trimethylcyclohexanone, it cannot be expected that the formation of cis alcohol should be exclusive because of the fact that the approach by the reducing species namely H− from the axial side is greatly hindered by two axial methyl groups (Tables 4 and 5). Reduction of 4-tert-butylcyclohexanone with NaBH4 under homogeneous condition yields ∼ 85% of the trans alcohol.36 The exclusive formation of trans-4-tert-butylcyclohexanonol in the present case can be rationalised only on the basis of nucleophilic activation by the silanol groups present on the surface of both acid treated calcined Si-MCM-41 and SBA-16 materials. It is expected that the silanol groups form hydrogen bonds with the carbonyl groups (Scheme 1a). Under such condition, the attack by H− from the equatorial side is blocked while H− adds up exclusively from the axial side. Since, 4-tert-butylcyclohexanone is a conformationally locked ketone, the molecule cannot flip and behaves like a rigid system. Under such circumstances, the H− has no other alternative but to attack from axial side resulting in the formation of 100% equatorial alcohol.37–39 Here, for hydride reductions of cyclic ketones, a four-centre transition state has been postulated.39 Thus, structures (Scheme 1b, I) and (Scheme 1b, II) represent at least qualitatively the transition states respectively for equatorial and axial attack of sodium borohydride on 4-tert-butylcyclohexanone. These transition states are compatible with the inverse isotope effect observed in boro-deuteride reduction and would also account for the well-known role which I-strain plays in hydride reduction. It has been reported that there is a considerable change in the degree of hybridisation of the C–O bond from sp2 to sp3 contrary to what one can infer from the Hammond postulate.40 It does not necessarily imply that the geometry of the transition state is tetrahedral (the strain in four-membered ring will be too much for that) which fact would explain the substantial absence of product stability control in the reductions. In case of 3-methylcyclohexanone, the reduction lead to the exclusive formation of cis-3-methylcyclohexanol in which the –OH group is equatorially disposed (Tables 4 and 5). 3-Methylcyclohexanone is also conformationally locked like 4-tert-butylcyclohexanone. The equatorial approach is severely blocked because of hydrogen bonding with the silanol groups from the active surface of Si-MCM-41 and SBA-16 materials. 3,3,5-Trimethylcyclohexanone is a sterically hindered ketone. The axial C3-methyl groups cause hindrance to any approaching nucleophile. Hence, the attack to the carbonyl group from the equatorial side is preferred. However, if there is substantial blockage towards approach by H− from the equatorial side, preferably the attack will take place from the axial side. In the present case, it appears that the complexation of the surface silanol groups with the carbonyl group is not that strong owing to the presence of an equatorially placed methyl group. Hence, exclusive formation of cis-3,3,5-trimethylcyclohexanol does not take place (Tables 4 and 5).
| Ketones (reduced) | Amounts used (mmol) | Chemical yields (%) of corresponding alcohols | Time of completion (h) |
|---|---|---|---|
| 4-tert-Butylcyclohexanone | (i) 2 | >99 (97) | 3 (7) |
| (ii) 5 | >99 (96) | 3 (7) | |
| (iii) 10 | >99 (94) | 3 (7.5) | |
| 2-Methylcyclohexanone | (i) 2 | 97 (86) | 3 (8) |
| (ii) 5 | 96 (85) | 3 (8.5) | |
| (iii) 10 | 95 (83) | 3.5 (9) | |
| 3-Methylcyclohexanone | (i) 2 | >99 (92) | 2 (6) |
| (ii) 5 | >99 (90) | 2 (6.5) | |
| (iii) 10 | >99 (89) | 2 (7) | |
| 4-Methylcyclohexanone | (i) 2 | 98 (94) | 3 (7) |
| (ii) 5 | 95 (90) | 3.5 (7) | |
| (iii) 10 | 91 (86) | 5 (8) | |
| 3,3,5-Trimethylcyclohexanone | (i) 2 | 97 (85) | 6 (9) |
| (ii) 5 | 94 (84) | 6.5 (9) | |
| (iii) 10 | 90 (82) | 7.5 (10) |
| Ketones (reduced) | Amounts used (mmol) | Chemical yields (%) of corresponding alcohols | Time of completion (h) |
|---|---|---|---|
| 4-tert-Butylcyclohexanone | (i) 2 | >99 (97) | 3 (7) |
| (ii) 5 | >99 (96) | 3 (7) | |
| (iii) 10 | >99 (94) | 3 (7.5) | |
| 2-Methylcyclohexanone | (i) 2 | 96 (86) | 3.5 (8) |
| (ii) 5 | 95 (85) | 3.5 (8.5) | |
| (iii) 10 | 93 (83) | 4 (9) | |
| 3-Methylcyclohexanone | (i) 2 | >99 (92) | 2 (6) |
| (ii) 5 | >99 (90) | 2 (6.5) | |
| (iii) 10 | 98 (89) | 2 (7) | |
| 4-Methylcyclohexanone | (i) 2 | 97 (94) | 3 (7) |
| (ii) 5 | 93 (90) | 4 (7) | |
| (iii) 10 | 89 (86) | 5.5 (8) | |
| 3,3,5-Trimethylcyclohexanone | (i) 2 | 96 (85) | 6.5 (9) |
| (ii) 5 | 92 (84) | 7 (9) | |
| (iii) 10 | 90 (82) | 8 (10) |
FTIR spectroscopic analysis (Fig. 4) was performed for the activated material–ketone complex (considering 4-tert-butylcyclohexanone). A perusal of Fig. 4 represents that the peak due to –OH (symmetrical stretching vibration, 3449 cm−1) is intense enough. Such increase in intensities points towards increase in surface activated silanol groups. A probable mechanism for opening up of siloxane (Si–O–Si) groups of the activated materials is that due to nucleophilic attack of Si atoms attached to ‘O’ atoms by OH− ions is suggested for increased surface active groups in case of acid treated activated solids (Fig. 4).
Determining the cause of the high activity for these materials requires understanding of the importance of their BET surface areas and the bulk morphology.41 It is well known that reducing activity is increased by larger surface area and larger pore volume along with ordered pore diameter.42 It has been noted that the enhancement of reduction for acid treated Si-MCM-41 is the highest, whereas acid treated SBA-16 is the lowest, compared to that previous one. The experimental data (Tables 2 and 5) that are consistent with the active materials being those with the largest surface area, larger pore diameter, and ordered mesostructure (Table 1). The reducing activity of acid treated calcined SBA-16 is lower mainly due to its low surface area (Table 1). Therefore, the material with the higher surface area, ordered pore diameter along with larger pore volume is favorable for better accessibility of the active sites, which helps to reduce those used ketone.
3.3. Reusability and recyclability of solid supports
We have attempted to investigate the recyclability of these materials. Therefore, we carried out calcination followed by treatment with concentrated HCl of these used solids and utilised for next reaction, which resulted in excellent yields along with reactivity of used ketones until the 5th time reuse (Fig. 5). XRD patterns and nitrogen sorption isotherms of spent materials (Fig. 6 and Table 6) have been carried out to check the effectiveness after five successive cycles. It has been observed from XRD patterns (Fig. 6a) that peak intensities remain almost the same as before. Only (210) peak has been diminished. Moreover, nitrogen adsorption–desorption isotherms (Fig. 6b) almost retain type IV isotherm as acid treated solids. From Table 6, it is clear that surface area (1187 m2 g−1 for acid treated calcined Si-MCM-41 and 663 m2 g−1 for acid treated calcined SBA-16), mean pore diameter (3.56 nm and 5.62 nm for acid treated calcined Si-MCM-41 and SBA-16, respectively), and overall pore volume (1.01 cm3 g−1 for acid treated calcined Si-MCM-41 and 0.89 cm3 g−1 for acid treated calcined SBA-16) have decreased a little after five consecutive cycles. So, it may be concluded that these active mesoporous silicas can act as highly active and reusable supports for reduction of cyclic ketones with preferential stereoselectivity.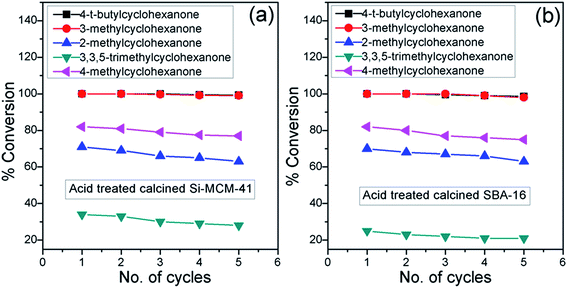 | ||
| Fig. 5 Conversion of different cyclic ketones in five successive cycles and reused with both acid treated Si-MCM-41 and SBA-16 materials. | ||
 | ||
| Fig. 6 XRD patterns (a) and nitrogen adsorption–desorption isotherms (b) of used materials after 5th cycle. | ||
| Materials | BET surface area (m2 g−1) | Average pore diameter (nm) | Total pore volume (cm3 g−1) |
|---|---|---|---|
| Cal. Si-MCM-41/Conc. HCl | 1187 | 3.56 | 1.01 |
| Cal. SBA-16/Conc. HCl | 663 | 5.62 | 0.89 |
4. Conclusion
In the present work, surface activated Si-MCM-41 and SBA-16 act as facile activators in the reduction of ketones. In some substituted cyclohexanones, the reductions are accompanied with good stereochemical outcome. Specially, in case of 4-tert-butylcyclohexanone and 3-methylcyclohexanone reduction using activated materials lead to exclusive formation of the epimer with equatorially disposed –OH group. Apparently, activated surface silanol groups activate the carbonyl group towards nucleophilic activation for reduction of the group. Furthermore, in the FTIR spectrum, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrational band of those used ketones generally appear near 1680–1720 cm−1. But, a perusal of Fig. 4 reveals that the corresponding ketone band moves to 1663 cm−1 due to the formation of the activated material–ketone complex. Such an observation clearly supports the hypothesis that the carbonyl groups are activated towards nucleophilic addition of H− from sodium borohydride during ketone reduction (Scheme 1). Acid treated calcined Si-MCM-41 material shows slightly higher reducing activity compared to the acid treated calcined SBA-16 material. Finally, it has been observed that these supports could exhibit excellent activity after five consecutive cycles of conversion and reuse.
O stretching vibrational band of those used ketones generally appear near 1680–1720 cm−1. But, a perusal of Fig. 4 reveals that the corresponding ketone band moves to 1663 cm−1 due to the formation of the activated material–ketone complex. Such an observation clearly supports the hypothesis that the carbonyl groups are activated towards nucleophilic addition of H− from sodium borohydride during ketone reduction (Scheme 1). Acid treated calcined Si-MCM-41 material shows slightly higher reducing activity compared to the acid treated calcined SBA-16 material. Finally, it has been observed that these supports could exhibit excellent activity after five consecutive cycles of conversion and reuse.
Acknowledgements
HC would like to thank Sophisticated Analytical Instrumentation Facility (SAIF), Punjab University, India for NMR facility.References
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- W. Shi, S. Tao, Y. Yu, Y. Wang and W. Ma, J. Mater. Chem., 2011, 21, 15567–15574 RSC.
- W. Long, N. A. Brunelli, S. A. Didas, E. W. Ping and C. W. Jones, ACS Catal., 2013, 3, 1700–1708 CrossRef CAS.
- E.-Y. Jeong, M. B. Ansari and S.-E. Park, ACS Catal., 2011, 1, 855–863 CrossRef CAS.
- R. Xing, N. Liu, Y. Liu, H. Wu, Y. Jiang, L. Chen, M. He and P. Wu, Adv. Funct. Mater., 2007, 17, 2455–2461 CrossRef CAS.
- T. Okada, Y. Takeda, N. Watanabe, T. Haeiwa, T. Sakai and S. Mishima, J. Mater. Chem. A, 2014, 2, 5751–5758 CAS.
- B. Karimi, H. M. Mirzaei, A. Mobaraki and H. Vali, Catal. Sci. Technol., 2012, 2, 828–834 CAS.
- B. Karimi, H. M. Mirzaei, A. Mobaraki and H. Vali, Catal. Sci. Technol., 2015, 5, 3624–3631 CAS.
- S. Shylesh, P. P. Samuel, C. Srilakshmi, R. Parischa and A. P. Singh, J. Mol. Catal. A: Chem., 2007, 274, 153–158 CrossRef CAS.
- G. Morales, G. Athens, B. F. Chmelka, R. Grieken and J. A. Merelo, J. Catal., 2008, 254, 205–217 CrossRef CAS.
- D. Das, J.-F. Lee and S. Cheng, J. Catal., 2004, 223, 152–160 CrossRef CAS.
- S. Miao and B. H. Shanks, J. Catal., 2011, 279, 136–143 CrossRef CAS.
- A. Corma, M. S. Grande, V. Gonzalez-Alfaro and A. V. Orichilles, J. Catal., 1996, 159, 375–382 CrossRef CAS.
- S. Bordiga, R. Buzzoni, F. Geobaldo, C. Lamberti, E. Giamello, A. Zecchina, G. Leofanti, G. Petrini, G. Tozzola and G. Vlaic, J. Catal., 1996, 158, 486–501 CrossRef CAS.
- S. Shylesh, P. P. Samuel, C. Srilakshmi, R. Parischa and A. P. Singh, J. Mol. Catal. A: Chem., 2007, 274, 153–158 CrossRef CAS.
- W. Long, N. A. Brunelli, S. A. Didas, E. W. Ping and C. W. Jones, ACS Catal., 2013, 3, 1700–1708 CrossRef CAS.
- B. Karimi, H. M. Mirzaei and A. Mobaraki, Catal. Sci. Technol., 2012, 2, 828–834 CAS.
- P. M. Price, J. H. Clark, K. Martin, D. J. Macquarrie and T. W. Bastock, Org. Process Res. Dev., 2015, 19, 872–877 CrossRef.
- H. C. Brown and S. Krishnamurthy, J. Am. Chem. Soc., 1972, 94, 7179 CrossRef.
- J. W. Huffman and W. W. McWhorter, J. Org. Chem., 1979, 44, 594–599 CrossRef CAS.
- S. Kim, K. H. Ahn and Y. W. Chung, J. Org. Chem., 1982, 47, 4581–4583 CrossRef CAS.
- G. Kovács, G. Galambos and Z. Juvancz, Synthesis, 1977, 171–172 CrossRef.
- N. G. Gaylord, J. Chem. Educ., 1957, 34, 367–374 CrossRef CAS.
- E. Schenker, Newer Methods of Preparative Organic Chemistry, ed. W. Foerst, Academic Press, New York, 1968, vol. IV, pp. 163–335 Search PubMed.
- A. Sarkar, H. Banichul and S. Saha, Energy Sources, Part A, 2012, 34, 465–477 CrossRef CAS.
- A. Sarkar and H. Banichul, Energy Sources, Part A, 2013, 35, 352–363 CrossRef CAS.
- Z. Qiao, L. Zhang, M. Guo, Y. Liu and Q. Huo, Chem. Mater., 2009, 21, 3823–3829 CrossRef CAS.
- G. Gu, P. P. Ong and C. Chu, J. Phys. Chem. Solids, 1999, 60, 943–947 CrossRef CAS.
- H. Chaudhuri, S. Dash and A. Sarkar, New J. Chem., 2016, 40, 3622–3634 RSC.
- G. Xuegui, S. Lei, W. Jianxin, H. Shaoyun and M. Guangwei, J. Rare Earths, 2005, 23, 521–525 Search PubMed.
- N. Pal and A. Bhaumik, RSC Adv., 2015, 5, 24363–24391 RSC.
- T. R. Pauly and T. J. Pinnavaia, Chem. Mater., 2001, 13, 987–993 CrossRef CAS.
- C. D. Wagner, D. E. Hilery, T. G. Kinisky, H. A. Six, J. F. Perez-Robles, R. Zamorano-Ulloa and J. Gonzalez-Hernandez, J. Phys. Chem. Solids, 2004, 65, 1045–1052 CrossRef.
- G. Leftheriotis, S. Papaefthimiou, P. Yianoulis, A. Siokou and D. Kefals, Appl. Surf. Sci., 2003, 218, 276–281 CrossRef.
- D. Nasipuri, A. Sarkar, S. K. Konar and A. Ghosh, Indian J. Chem., 1982, 21, 2840–2845 Search PubMed.
- W. G. Dauben, G. J. Fonken and D. S. Noyce, J. Am. Chem. Soc., 1956, 78, 2579–2582 CrossRef CAS.
- A. Sarkar and B. R. Rao, Tetrahedron Lett., 1991, 32, 1247–1250 CrossRef CAS.
- P. Geneste and G. Lamaty, Tetrahedron Lett., 1965, 51, 4633–4638 CrossRef.
- J. S. Hammond, J. Am. Chem. Soc., 1955, 77, 334–338 CrossRef.
- M. Kruk, M. Jaroniec, C. H. Ko and R. Ryoo, Chem. Mater., 2000, 12, 1961–1968 CrossRef CAS.
- Z. Liu, E. Lindner and H. A. Mayer, Chem. Rev., 2002, 102, 3543–3578 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21480k |
| This journal is © The Royal Society of Chemistry 2016 |