Rapid pre-symptomatic recognition of tristeza viral RNA by a novel fluorescent self-dimerized DNA–silver nanocluster probe†
Abstract
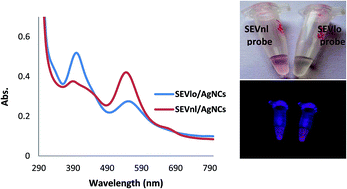
Citrus tristeza virus (CTV), a positive-strand RNA virus within the family of Closteroviridae, is distributed worldwide and causes one of the most economically important diseases of citrus. Since the CTV pathogen is easily spread by graft propagation and aphid vectors, continual monitoring of healthy seedlings and eradication of infected plants is essential for better disease management. In this study, a novel self-dimerized DNA–silver nanocluster probe was developed for the simple and rapid pre-symptomatic detection of CTV severe strains in biological preparations. Insertion of a G rich loop maker sequence containing internal complementary bases in the probe structure leads to the formation of a stable homodimer probe with a G-rich loop at the center that is more reactive than the formless probe. Interestingly, the results showed that the probe structure conversion between dimeric and non dimeric states upon DNA/RNA hybridization, produced an enhanced fluorescence signal allowing precise and sensitive detection of tristeza RNA. Based on the sensitivity test, CTV-RNA in the range of 5–210 nM, can be linearly detected with the detection limit of 2.5 nM. Finally, the results of our DNA–AgNCs based fluorometric assay were consistent with the results of the conventional RT-PCR.

 Please wait while we load your content...
Please wait while we load your content...