Combined pretreatment of lignocellulosic biomass by solid base (calcined Na2SiO3) and ionic liquid for enhanced enzymatic saccharification
Abstract
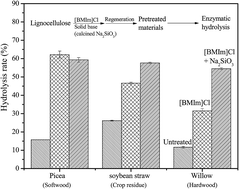
A novel method combining a solid base catalyst (Na2SiO3) and a cheaper ionic liquid (1-butyl-3-methylimidazolium, [BMIm]Cl), was proposed and used for the pretreatment of lignocellulosic biomass such as spruce, willow and soybean straw. The addition of calcined Na2SiO3 in [BMIm]Cl pretreatment significantly destroyed the recalcitrant cell wall architecture, removed lignin and hemicellulose, decreased cellulosic crystallinity, and strongly broke lignocellulosic morphology, which enhanced the biomass accessibility for enzymatic hydrolysis. The combined pretreatment seemed more suitable for willow and soybean straw than spruce. According to single factor experiments, the maximum enzymatic hydrolysis yield and glucose yield of willow were 98.6% and 39.5 g/100 g biomass, respectively, 2.6-fold of single pretreatment with [BMIm]Cl.

 Please wait while we load your content...
Please wait while we load your content...