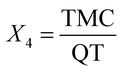Co-encapsulation of tamoxifen citrate and quercetin using 2HP-β-cyclodextrin: a response surface experimental design†
Fatemeh Zarei baygia,
Nafiseh Farhadian*a,
Bizhan Malaekeh-Nikoueib and
Zahra Maghsouda
aChemical Engineering Department, Faculty of Engineering, Ferdowsi University of Mashhad, Mashhad 9177948974, Iran. E-mail: n.farhadian@um.ac.ir
bNanotechnology Research Center, School of Pharmacy, Mashhad University of Medical Sciences, I. R. Iran
First published on 10th November 2016
Abstract
The aim of this study is to prepare a nanosphere containing the insoluble anti-cancer drug called tamoxifen citrate. The synthesized nanosphere is composed of tamoxifen citrate (TMC), 2-hydroxy propyl-β-cyclodextrin (2HP-β-CD), quercetin (QT) and polycaprolactone (PCL), which was prepared using water in oil in water emulsion solvent extraction method. 2HP-β-CD and QT were applied to enhance the solubility and bioavailability of the drug, respectively. The response surface method was used to optimize the operating variables such as PCL polymer mass, stabilizer percentage, continuous water volume and 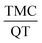 ratio based on the central composite design. Quadratic models were developed for two responses: the drug encapsulation efficiency and the nanosphere size. According to the results, the optimum conditions to achieve a nanosphere of a small size and high drug entrapment efficiency included 30 mg PCL, 2% (w/v) PVA stabilizer, 80 ml water and
ratio based on the central composite design. Quadratic models were developed for two responses: the drug encapsulation efficiency and the nanosphere size. According to the results, the optimum conditions to achieve a nanosphere of a small size and high drug entrapment efficiency included 30 mg PCL, 2% (w/v) PVA stabilizer, 80 ml water and 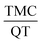 equivalent ratio of 0.5. According to the UV analysis based on the second derivative spectrophotometric technique, the estimated encapsulation efficiency of TMC was 53% ± 2. Scanning electron microscopy (SEM) showed that the mean particle size of the synthesized nanospheres was 205 nm, which is appropriate for oral usage. In addition, the in vitro release of TMC and QT in the simulated body environment (SGF and SIF) was performed at 37 °C. The in vitro release of TMC at pH = 7.4 showed that TMC and QT had a slow and simultaneous release behaviour. This confirms the controllable release of TMC in the new synthesized nanoparticle form.
equivalent ratio of 0.5. According to the UV analysis based on the second derivative spectrophotometric technique, the estimated encapsulation efficiency of TMC was 53% ± 2. Scanning electron microscopy (SEM) showed that the mean particle size of the synthesized nanospheres was 205 nm, which is appropriate for oral usage. In addition, the in vitro release of TMC and QT in the simulated body environment (SGF and SIF) was performed at 37 °C. The in vitro release of TMC at pH = 7.4 showed that TMC and QT had a slow and simultaneous release behaviour. This confirms the controllable release of TMC in the new synthesized nanoparticle form.
1. Introduction
Cancer is a fatal disease and a primary cause of death in the world. Breast cancer is the most common malignant cancer in women.1 The chance of developing an invasive breast cancer in a woman is approximately 1 in 8 (∼12%).2 Considering that breast cancer is fatal in 35% of cases,3 it is necessary to find an efficient method to treat breast cancer. To achieve this end, various drugs have been used. One of the most effective drugs for the treatment and prevention of breast cancer is tamoxifen citrate.4Tamoxifen citrate is a selective oestrogen receptor antagonist oral drug.5,6 In patients with breast cancer, the anti-oestrogen effects of the breast tissue have a simultaneous effect on other tissues such as bone,7 liver,8 endometrial,9 and cardiovascular system.10 As a result, there is a competition between tamoxifen and estradiol for the oestrogen receptor, which prevents the binding of estradiol to the receptor. This mechanism can treat breast cancer.11 Moreover, after primary treatment, tamoxifen can be used to reduce the risk of an invasive breast cancer for 10 years.12 The long-time usage of this oral drug can produce a number of serious side effects, such as hot flashes, fatigue, toxic hepatitis, multifocal hepatic fatty infiltration, sub massive hepatic necrosis and cirrhosis as well as increased risk of blood clots and oxidative stress mediated hepatotoxicity.13 Furthermore, tamoxifen increases the risk of endometrial cancer, which is mainly dependent upon the treatment duration and dose accumulation.9 Thus, lower drug doses with higher selective adsorption would be more effective.8 One of the best strategies to enhance drug adsorption efficiency is to reduce the size of drug carrier as it increases the contact surface area of the drug with the surrounding environment. This enhances the therapeutic effect of drug and thus lowers the dose usage. It has been shown that some of the side effects of this drug can be reduced.14
In addition to general side effects of chemotherapeutic drugs, each drug may have some specific problems in relation to its consumption. For TMC, two main problems have been reported, including its low solubility in the hydrophilic environment of body due to its hydrophobic nature, and its low bioavailability and insufficient adsorption in the gastrointestinal tract and removal by the first-pass liver metabolism.8 To overcome the first problem, some compounds with hydrophobic–hydrophilic nature have been suggested. In this regard, 2-hydroxy propyl-β-cyclodextrin (2HP-β-CD) is one of the best candidates owing to its high solubility in aqueous environments and low toxicity. Also, producing a complex containing TMC and 2HP-β-CD is rather simple.15,16 Shukla et al., formulated and characterized a water-soluble complex of TMC-2HP-β-CD to enhance TMC solubility. They found that TMC-2HP-β-CD nanoassembly infiltrated the tissue due to the enhanced permeability and retention property of tumour tissue. This produced excellent anticancer effect in the ovarian cancer cell line.17 In another study, 2HP-β-CD was applied to enhance bioavailability and solubility of tamoxifen citrate. Results showed the low level of synthesized nanoparticles in plasma and liver in comparison to oral TMC suspension.18
The second problem of TMC consumption as low bioavailability is related to the aromatase enzyme function. Aromatase is the enzyme that catalyzes the conversion of androstenedione and testosterone to oestrogen and estradiol.19 Tamoxifen citrate, inhibiting the function of this enzyme, reduces the amount of estradiol.17 Applying aromatase inhibitors to the TMC formulation may decrease drug resistance in the breast cancer and enhance bioavailability.8 The application of flavonoids, due to special phenolic structure, can prevent injury caused by free radicals, which may lead to cell damage,20 aging and stroke.21 Quercetin (QT) is a flavonoid with high therapeutic effects, which has been used with TMC in literature.8,18 Caltagirone et al. showed that TMC and quercetin were effective in inhibiting in vitro. Also, TMC was found to compete with quercetin in whole-cell assays for type-II binding, thus inhibiting cell proliferation in a dose-dependent manner in a multidrug-resistant, ER-negative MCF-7 breast cancer cell line.22 In another study, a combination of quercetin and tamoxifen was shown to be more effective in reducing cell viability than tamoxifen and quercetin separately. In addition, TMC-QT complex led to a significant inhibition of cell viability by decreasing Bcl-2 protein expression and inducing apoptosis.23 Jain et al. showed that free TMC and QT had 2% (ref. 8) oral bioavailability in humans, which is a low value. The co-administration of quercetin enhanced the oral bioavailability of TMC by 5.31-fold.8
A review of the literature suggests that both 2HP-β-CD and QT have been applied to TMC formulation separately. However, the co-administration of TMC with both TMC and QT has not been reported. The main goal of this study is to provide a co-encapsulation of TMC with 2HP-β-CD and QT in a nanostructure form. To do so, nanosphere was selected as an appropriate form of drug carrier at nanometre scale. In oral delivery, polyester-based NPs could protect the drug against the gastrointestinal (GI) harsh environment and enhance the oral adsorption of drug with systemic targeting by avoiding the intravenous route.24 Thus, this type of carrier was adopted in this study. Also, polycaprolactone (PCL) was chosen as the appropriate polymer. PCL has a slow degradation rate in comparison to other polymers such as poly(lactic-co-glycolic acid) (PLGA),25 polylactic acid (PLA) and polyglycolide (PGA)26 is slow. Hence, PCL nanosphere could be a suitable carrier for long-term drug release systems.27,28 According to previous research, the oral bioavailability of tamoxifen citrate was significantly enhanced by its encapsulation in PCL NPs, leading to specific immunosuppression without increased adverse effects.24 Finding the best nanosphere structure with the appropriate size, drug loading and drug entrapment efficiency is a critical consideration in the design of drug delivery systems. In this study, the design expert method using response surface methodology was adopted to optimize the preparation parameters. Then, the best structure of the drug release was examined in experimental environments at pH = 7.4 and simulated gastric and intestinal fluid. Designing a best structure for slow and simultaneous release of TMC and QT is the main novelty of this research.
2. Materials and methods
2.1 Materials
Tamoxifen citrate was received as a gift sample from Iranian Hormone Pharmaceutical Company, and quercetin, polycaprolactone (MW = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), 2-hydroxypropyl beta cyclodextrin and polyvinyl alcohol (PVA, MW: 70
000), 2-hydroxypropyl beta cyclodextrin and polyvinyl alcohol (PVA, MW: 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–100
000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, powder, 98% hydrolyzed) were purchased from Sigma-Aldrich. Dichloromethane (DCM), Tween 80, NaOH, KH2PO4, HCL and NaCl were purchased from Merck Company.
000, powder, 98% hydrolyzed) were purchased from Sigma-Aldrich. Dichloromethane (DCM), Tween 80, NaOH, KH2PO4, HCL and NaCl were purchased from Merck Company.
2.2 Preparation of TMC-β-CD inclusion complex
The inclusion complex of tamoxifen citrate was prepared using 2HP-β-CD at different ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio of TMC to 2HP-β-CD) as follows:
3 molar ratio of TMC to 2HP-β-CD) as follows:
Tamoxifen citrate (3.54 mM) and 2HP-β-CD (3.54, 7.1, 10.62 mM) were mixed in 2 ml of distilled water, stirred (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 24 h) at 25 °C and filtered through a 0.45 μm membrane filter to remove undissolved material. Then, the TMC content was determined by UV spectroscopy (UV-FP-6200, JASCO Instruments, Japan) at a wavelength of λ = 237 nm. The inclusion efficiency (%) was calculated using the following formula.17,29
000 rpm for 24 h) at 25 °C and filtered through a 0.45 μm membrane filter to remove undissolved material. Then, the TMC content was determined by UV spectroscopy (UV-FP-6200, JASCO Instruments, Japan) at a wavelength of λ = 237 nm. The inclusion efficiency (%) was calculated using the following formula.17,29
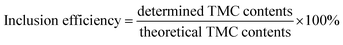 | (1) |
Furthermore, the extinction coefficient of drug was calculated using eqn (2) as follows:
| A = εCL | (2) |
2.3 Preparation of TMC nanosphere
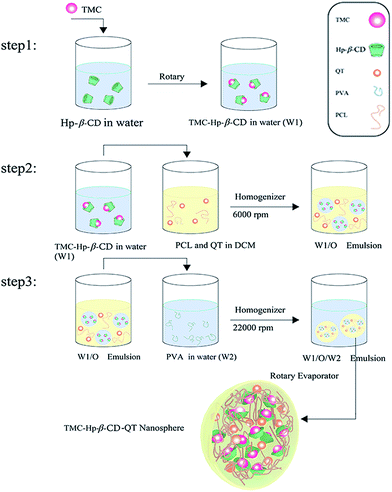
TMC nanosphere was prepared using W/O/W emulsion solvent extraction method. The internal aqueous phase (W1) contains 2 ml aqueous solution of TMC-2HP-β-CD complex (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 TMC to 2HP-β-CD molar ratio). W1 was emulsified by a homogenizer of 6000 rpm (model IKA-T10 Heidolph, Germany) for 5 min into a 10 ml solution of dichloromethane (containing 30 mg of PCL and 3 mg QT). The primary W/O emulsion was dispersed by the ultrasonic bath and added drop-wise to 60 ml of 3% (w/v) PVA aqueous solution, while stirred by a homogenizer of 22
2 TMC to 2HP-β-CD molar ratio). W1 was emulsified by a homogenizer of 6000 rpm (model IKA-T10 Heidolph, Germany) for 5 min into a 10 ml solution of dichloromethane (containing 30 mg of PCL and 3 mg QT). The primary W/O emulsion was dispersed by the ultrasonic bath and added drop-wise to 60 ml of 3% (w/v) PVA aqueous solution, while stirred by a homogenizer of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (model Diax 900 Heidolph, Germany) for 10 min. Nano-particle suspension was obtained after the evaporation of solvent under dropped pressure using a rotary evaporator (Evaporator, Heidolph, Germany).
000 rpm (model Diax 900 Heidolph, Germany) for 10 min. Nano-particle suspension was obtained after the evaporation of solvent under dropped pressure using a rotary evaporator (Evaporator, Heidolph, Germany).
Finally, the nanospheres were separated from the bulk suspension, and the free drug was removed by centrifugation. The supernatant (liquid layer above the sediment) was maintained for drug assay with the sedimented nanospheres at the bottom of falcon tube being washed three times with deionized water in successive centrifugation/redispersion cycles for 20 min at a temperature of 25 °C. Finally, nanoparticles were freeze-dried.30,31 All experiments were repeated 3 times to confirm the observed trends. In Scheme 1, the schematic of applied procedure for nanosphere synthesis has been shown.
2.4 Derivative spectrophotometric method
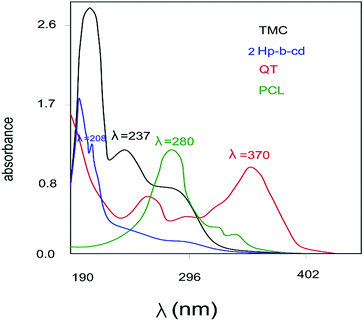
TMC and QT concentrations in polymeric particles were determined using spectrophotometrically analysis (UV-FP-6200, JASCO Instruments, Japan). Fig. 1 shows the UV spectra of all components presented in the nanosphere. Four wavelengths of 237 nm,32 370 nm,33 280 nm (ref. 34) and 208 nm (ref. 35) were selected for TMC, QT, PCL and 2HP-β-CD measurement, respectively. As shown in Fig. 1, the QT concentration can be estimated simply by UV spectra. For calculation of TMC concentration at 237 nm, however, there is an overlapping with the spectra of other components. Considering different methods proposed for evaluation of this overlapping, an analytical technique is required for the simultaneous analysis of the drug mixtures.36 This study presents an inexpensive method for simultaneous determination of components in the synthesized nanosphere using derivative ratio spectra zero-crossing method.37 In zero-crossing method, for each component presented in the nanosphere, the UV spectra curve will be differentiated until a fixed wavelength with the UV spectra curve of a special component becoming independent of other components. Then, this new wavelength is used to calculate the concentration of that certain component. The optimum wavelength is selected for TMC with respect to the fact that the contribution of each component will be zero at the wavelength in which the maximum absorbance of TMC is exhibited.A first and second derivative spectrum of each component were subsequently recorded (see ESI file, Fig. S1†). As shown in Fig. S1,† in the second derivative curve, the simultaneous determination of TMC can be performed at a wavelength of 215 nm. This new wavelength was selected to calculate TMC concentration in the nanosphere form. The calibration curves of TMC and QT are presented in the ESI file (Fig. S2–S4†). It should be noted that by using UV visible analysis, it is possible to measure TMC content in the free form not in the encapsulated form by CD. Moreover, at the applied wavelength for TMC measurement, other components have the zero contribution.
2.5 Particle size analysis
Dynamic light scattering was used to evaluate the size of the W/O/W emulsion dispersed in deionized water as well as polydispersity index using a Particle Size Analyser (Nano-zs, Malvern Instruments, UK). Moreover, z-potential was measured using the aforementioned equipment. Both measurements were carried out at 25 °C using a laser beam with a wavelength of 633 nm. The scattering angel was 90° and the particle concentration was in the range of 0.15 to 0.2 ppm.2.6 Particle morphology
Scanning Electron Microscope (SEM) was used to characterize surface and shape morphology. The nanospheres were first freeze-dried and the powder was fixed on supports of carbon-glue coated with gold palladium under an argon atmosphere using a gold sputter module in a high-vacuum evaporator. Samples were then observed by a scanning electron microscope (Hitachi S-4160, Japan) at 20 kV.2.7 Experimental design
To improve the quality of statistical experiments, the response surface methodology (RSM) was adopted. This is a widely used approach for the improvement and optimization of drug delivery systems.38 To do so, a 4-factor, 4-level respond design including PCL mass (X1), % PVA (X2), continuous phase volume (X3) and TMC to QT ratio as the independent variables was used to optimize nanosphere formulation. The low and high levels of variables have been described in Table 1. The dependent variables were selected as particle size (Y1) and TMC entrapment efficiency (Y2). Design-Expert software (v.7.1.5 Stat-Ease Inc., Minneapolis, USA) was used for the generation and evaluation of the statistical experimental design.| Dependent variables | Constraints |
|---|---|
| Y1 = particle size (nm) | Minimum |
| Y2 = entrapment efficiency of TMC (%) | Maximum |
2.8 Drug release studies
The TMC and QT loaded in the nanosphere were released at a temperature of 37 °C at pH = 7.4 phosphate buffered saline (S) which contained Tween 80 (2.5% w/v). In brief, the particles in suspension were gently stirred at 37 °C into a shaker incubator (100 rpm). The suspension was fully withdrawn at specific intervals, every day up to 17 days. Each sample was centrifuged at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The filtrate was replaced by 2 ml of fresh buffer.39 The amounts of TMC and QT in the released medium were determined by UV Vis analysis at 215 nm for TMC and 370 nm for QT using eqn (3):
000 rpm for 10 min. The filtrate was replaced by 2 ml of fresh buffer.39 The amounts of TMC and QT in the released medium were determined by UV Vis analysis at 215 nm for TMC and 370 nm for QT using eqn (3):
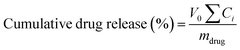 | (3) |
2.9 Drug release studies in the simulated gastric and intestinal fluid
The in vitro release patterns of both TMC and QT drugs from synthesized nanosphere were evaluated in simulated gastric fluid without pepsin (SGF) and enzyme-free simulated intestinal fluid (SIF). Thus, drug release were studied in SGF (HCl/NaCl solution containing 0.02% Tween 80, pH = 1.2) for 2 h. Then, the dissolution medium was replaced with enzyme-free SIF (KH2PO4/NaOH buffer containing 0.02% Tween 80, pH = 7.4) and tested for drug release study for 3 h. Finally enzyme-free SIF (KH2PO4/NaOH buffer containing 0.02% Tween 80, pH = 6.5) was used for 7 h to mimic colonic pH conditions.40The particles suspension was gently stirred at 37 °C in a shaker incubator (100 rpm). The final suspension was withdrawn at appropriate intervals (0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10 and 12 h) with each sample being centrifuged at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The filtrate was replaced by 2 ml of fresh buffer. The content of tamoxifen citrate and quercetin in the release medium were determined by UV Vis analysis. It should be noted that all experiments were repeated three times at each stage to achieve optimal evaluation.
000 rpm for 10 min. The filtrate was replaced by 2 ml of fresh buffer. The content of tamoxifen citrate and quercetin in the release medium were determined by UV Vis analysis. It should be noted that all experiments were repeated three times at each stage to achieve optimal evaluation.
3. Results and discussion
3.1 Preparation of inclusion complex
Fig. 2 shows the inclusion efficiency variation for different molar ratios of TMC to 2HP-β-CD as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. As can be seen, the maximum inclusion efficiency occurs at a ratio of 1
3. As can be seen, the maximum inclusion efficiency occurs at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of TMC to 2HP-β-CD. This finding is in agreement with previous studies.17,18
2 of TMC to 2HP-β-CD. This finding is in agreement with previous studies.17,18
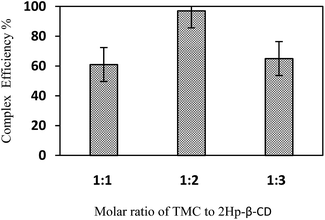 | ||
| Fig. 2 The inclusion efficiencies of TMC and 2HP-β-CD mixture at different molar ratios. Each data point represents mean ± SD (n = 3). | ||
Moreover, the extinction coefficient of drug before and after encapsulation with β-CD indicated that the ratio of εafter encapsulation with β-CD/εbefore encapsulation with β-CD was 0.34. This demonstrates a reduction in the extinction coefficient of drug, which can affect the release rate of TMC from nanosphere. It will be discussed in details later.
3.2 Response surface analysis
The response surface analysis was selected 25 runs for 4 independent variables according to Table 1. For each variable group, nanosphere synthesis was applied and the particle size and entrapment efficiency of TMC were measured as dependent variables. It should be noted that the particle size was reported based on DLS analysis using intensity curves. The formulation factors and corresponding observations of dependent variables are presented in Table 2. As shown in Table 2, the size of the nanospheres varies from 120 nm up to 350 nm. The 3D graph of particle size versus independent factors is illustrated in Fig. 3. Fig. 3(a) shows that an increase in the polymer amount enhances the particle size. It is clear that by increasing the polymer amount, the viscosity of the dispersed phase increases. This increases the diffusional resistance of drug molecules in the organic phase compared to the aqueous phase, thereby causing the entrapment of a large number of drug in the polymer nanosphere. This increases the nanosphere size and encapsulation efficiency (Fig. 4a–c), which is in agreement with previous studies.41,42| Run | Independent variable | Y1 (nm) | Y2 (%) | |||
|---|---|---|---|---|---|---|
| X1 (mg) | X2 (%) | X3 (ml) | X4 (—) | |||
| 1 | 40.00 | 1.00 | 80.00 | 0.75 | 250 | 50 |
| 2 | 50.00 | 1.00 | 60.00 | 0.50 | 300 | 60 |
| 3 | 60.00 | 2.00 | 80.00 | 0.75 | 350 | 70 |
| 4 | 40.00 | 2.00 | 80.00 | 0.75 | 270 | 53 |
| 5 | 30.00 | 1.00 | 60.00 | 1.00 | 180 | 40 |
| 6 | 50.00 | 3.00 | 100.00 | 0.50 | 310 | 64 |
| 7 | 40.00 | 2.00 | 40.00 | 0.75 | 340 | 52 |
| 8 | 50.00 | 3.00 | 60.00 | 1.00 | 336 | 58 |
| 9 | 50.00 | 3.00 | 100.00 | 1.00 | 300 | 55 |
| 10 | 30.00 | 1.00 | 100.00 | 1.00 | 150 | 44 |
| 11 | 50.00 | 1.00 | 100.00 | 1.00 | 310 | 65 |
| 12 | 50.00 | 1.00 | 100.00 | 0.50 | 280 | 63 |
| 13 | 50.00 | 3.00 | 60.00 | 0.50 | 326 | 64 |
| 14 | 30.00 | 1.00 | 100.00 | 0.50 | 145 | 43 |
| 15 | 40.00 | 2.00 | 80.00 | 1.25 | 295 | 54 |
| 16 | 30.00 | 3.00 | 100.00 | 1.00 | 190 | 50 |
| 17 | 40.00 | 2.00 | 120.00 | 0.75 | 274 | 53 |
| 18 | 30.00 | 1.00 | 60.00 | 0.50 | 165 | 47 |
| 19 | 30.00 | 2.00 | 80.00 | 0.50 | 205 | 53 |
| 20 | 40.00 | 2.00 | 80.00 | 0.25 | 220 | 59 |
| 21 | 40.00 | 4.00 | 80.00 | 0.75 | 296 | 60 |
| 22 | 20.00 | 2.00 | 80.00 | 0.75 | 120 | 43 |
| 23 | 30.00 | 3.00 | 60.00 | 0.50 | 185 | 52 |
| 24 | 50.00 | 1.00 | 60.00 | 1.00 | 320 | 60 |
| 25 | 30.00 | 3.00 | 100.00 | 0.50 | 176 | 48 |
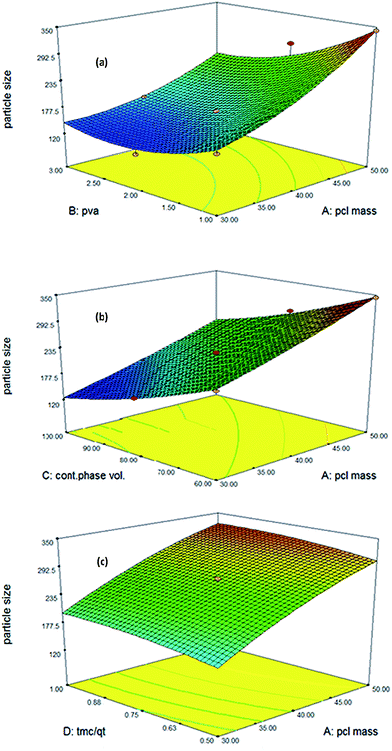 | ||
Fig. 3 Response surface plot showing the effect of (a) PCL mass (X1) and % PVA (X2), (b) % PVA (X2), and continues phase volume (X3), (c) 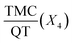 and PCL mass (X1). and PCL mass (X1). | ||
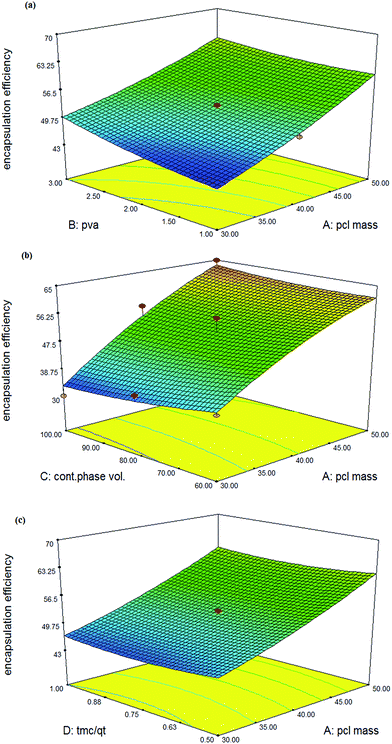 | ||
Fig. 4 Response surface plot showing the effect of (a) PCL mass (X1) and % PVA (X2), (b) % PVA (X2), and continues phase volume (X3), (c) 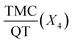 and PCL mass (X1). and PCL mass (X1). | ||
Also, the particle size is increased as a result of reduction in the continuous phase volume. With a reduction in the volume of continuous phase molecules, drugs stick together and produce larger nanosphere. An increase in the tension force between dispersed and continuous phases increases the particle size (Fig. 3b) and encapsulation efficiency. However any change in the volume of continues phase exerts a significant effect on the particle size in comparison to encapsulation efficiency (Fig. 4b).43 Furthermore, an increase in the concentration of surfactant (% PVA) can reduce the size of nanospheres.
Applying surfactant as a stabilizer in the emulsification stage diminishes the surface tension of the continuous phase, resulting in a significant hike in the net shear stress at a constant energy density. This promotes the formation of small-size emulsion droplets and thus decreases the mean diameter of nanospheres.44
According to our observations, there is an optimum percentage for the surfactant concentrations. Applying 1 to 2% of the PVA leads to a significant decrease in the nanosphere size. However, increasing PVA concentration from 2 to 3% (p < 0.05) produces a plateau trend in the particle size curve. This may be related to the saturation of the particle surfaces by emulsifiers. This confirms that an increase of more than 2% in the emulsifier concentration will not have any effect on the particle size (Fig. 3a and d), with a decrease in the particle size from 1 to 2% PVA reducing the extent of encapsulation efficiency (Fig. 4a).
According to Fig. 3(c), no significant relationship was observed between particle size and TMC to QT ratio.
The statistical analysis of the experimental data was used to identify the best fitting models for the independent variables. The quadratic model for particle size (R2 = 0.981) and the entrapment efficiency of TMC (R2 = 0.982) and QT (R2 = 0.979) was established. The fitting equations obtained for the particle size and encapsulation efficiency were as follows:
| Particle size = 284.8 + 63.92X1 + 15.57X2 − 12.5X3 + 10.5X4 − 15.42X12 − 11.41X22 − 9.79X42 | (4) |
| Encapsulation efficiency = 52.40 + 7.33X1 + 2.1X2 + 0.083X3 − 0.33X4 − 1.12X1X2 + 1.38X1X3 + 0.63X3X4 + 1.03X12 + 0.79X22 + 1.03X24 | (5) |
Finally, the optimum nanosphere size to achieve the small particle size and high encapsulation efficiency simultaneously was formulated as containing 30 mg PCL, 80 ml of continuous phase volume (w2), 2% PVA and a TMC to QT ratio 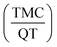 of 0.5. Under optimum conditions, the particle size is estimated as 205 with a TMC encapsulation and entrapment of 53%.
of 0.5. Under optimum conditions, the particle size is estimated as 205 with a TMC encapsulation and entrapment of 53%.
3.3 Measurement of particle size and morphology
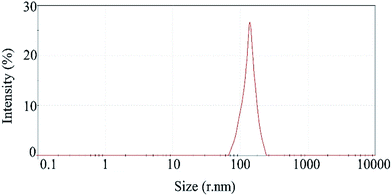
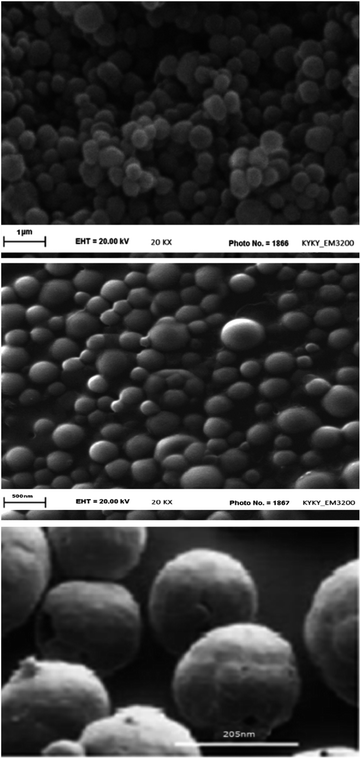
Fig. 5 shows the results of DLS analysis for the synthesized nanosphere using the optimum conditions derived from the statistical results. Figure shows that the particle size of nanosphere is about 205 ± 2 nm (data are expressed as mean ± SD (n = 3)) with the PDI value 0.24 ± 0.05. The z-potential was measured as +20 for the synthesized nanosphere. The spherical shape of nanospheres was approved using SEM analysis. According to SEM images, synthesized nanospheres are spherical and polydispersed with a diameter of 200–205 nm (Fig. 6).3.4 Encapsulation efficiency
Encapsulation efficiency and drug loading can be calculated based on eqn (6) and (7):
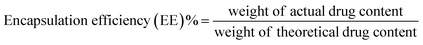 | (6) |
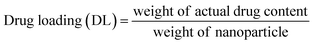 | (7) |
Using eqn (6) and (7), the encapsulation efficiency was estimated as 53 ± 2% for TMC and 55 ± 2% for QT. In addition, the estimated drug loading was 10.6 ± 0.1% for TMC and 11 ± 0.1% for QT.
3.5 Drug release
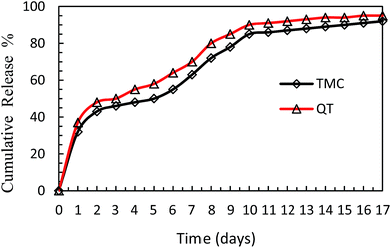
The in vitro release profile of TMC and QT from nanosphere at pH = 7.4 are shown in Fig. 7. As shown in Fig. 7, about 32% of TMC and 38% of QT were released in the first 24 h, followed by a sustained release lasting for 17 days. After 17 days, about 90% of TMC and 94.23% of QT had been released from the nanosphere, whereas almost 100% release of both TMC and QT was observed within 1 h in free form.8This observation confirms the long-term delivery of drugs from nanosphere. Moreover, TMC and QT were released from the nanosphere at a similar rate. This can improve the bioavailability of TMC and decrease some side effects of TMC tablets, which is consistent with the findings of Jain8 and Diab.24
This controllable release of drugs from nanosphere can be attributed to the slow application of degradable PCL in the polymeric matrix.25 The drug release from biodegradable polymers is controlled by three mechanisms: Fickian diffusion through the polymer matrix, diffusion through pores in the matrix, and drug liberation by polymer erosion.39 The slow PCL degradation compared to poly glycolic acid and other polymers makes it suitable for long-term delivery greater than a year.45,46 Given the slow degradation of PCL in aqueous environments due to its semi-crystalline and hydrophobic nature, the drug release from synthesized NP may be due to diffusion mechanism no the polymer degradation.47
This slow release of TMC from nanosphere structure is in agreement with previous studies. Jain K. et al. prepared a nanosphere structure containing TMC drug in a polymeric matrix using PLGA instead of PCL in the absence of β-CD usage.8 The release of TMC from their NP (TMC–PLGA) lasted for 20 days, which is a long term delivery for the drug. It should be noted that the difference between the structure proposed by Jain K et al. and our nanosphere release time can be attributed to different nature of applied polymers (PLGA and PCL).8 Moreover, applying β-CD to our structure can enhance drug release in aqueous environments.48 In other words, water penetration into the PCL polymer matrix in the present of β-CD is easier than other corresponding structures.
The sustained release of TMC at pH = 7 shows that this drug can be a good candidate for injectable usage compared to orally administrated drugs, but the convenient and cost effective route of drug usage is the oral route. In some cases, this drug is prescribed in a dosage of 20 mg per day for a period of at least 5 to 10 years. The oral administration for this long time period is the best method. In addition, since the main goal of this study was to improve the solubility and bioavailability of TMC in the commercial form (tablet), the injectable usage of this drug was not considered.
3.6 Drug release in SGF and SIF conditions
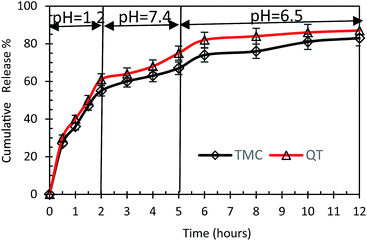
Fig. 8 shows the release profile of TMC and QT under SGF and SIF conditions. As can be seen, the drug release in the acidic environment of the stomach is burst. Nearly 55% of TMC and 60% of QT were released within 2 h under SGF condition (pH = 1.2). Considering the weak base of tamoxifen (pKa: 8.8),49 its ionization will occur in the gastric environment, leading to a predictably rapid dissolution in the stomach. As the drug passes through the stomach to the small intestinal, its dissolution is slowed as a result of significant pH enhancement (pH = 7.4) and reduced drug ionization.50 The drug release continues in the small intestine and is terminated in the large intestine (colon) (pH = 6.5).According to previous studies, the release rate of TMC in tablet form was 80% in SGF and 45% in SIF environments after 10 min.50 A comparison of the results of this study with previous results confirms that the release of TMC as a synthesized nanosphere in SGF and SIF environments was slower than its usual form. The slow release of TMC in the proposed form may enhance its bioavailability for long-term usage in patients with breast cancer.51
Similar to the PBS environment (pH = 7.4), the mechanism through which drug is released from nanosphere in SGF and SIF environments is diffusion. According to the previous studies,26 PCL microparticles are resistant to the simulated gastric fluid and can protect adjuvant and/or the antigen from proteolytic destruction in the stomach. This allows the entrapped drugs to pass into the intestine for the presentation of the gut-associated lymphoid system.
Moreover, as shown in Fig. 7 and 8, TMC is not released from the nanosphere completely. This may be due to the reduced extinction coefficient of TMC caused by encapsulation in β-CD. As discussed above, the calculated values of TMC by UV visible analysis is related to the free form of TMC not encapsulation form. In the other word, some TMC content remains in β-CD and could not be released in the buffer medium due to strong interactions between TMC and CD surface.
4. Conclusions
In this study, a new formulation of tamoxifen citrate drug was proposed to improve the solubility and bioavailability of the tamoxifen citrate in the aqueous environment. TMC nanosphere was synthesized by emulsion solvent evaporation method. 2HP-β-CD and quercetin were applied to increase the solubility and bioavailability of the drug, respectively. Derivative spectrophotometric method was successfully used to predict the content of drugs in nanoparticles. The optimization of some operating parameters such as PCL mass, % PVA, continues phase volume and TMC to QT ratio were performed using response surface method. According to the experimental results, the estimated optimum molar ratio of TMC to 2HP-β-CD was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. This ratio was applied to prepare nanosphere at the initial aqueous phase. The results of response surface analysis suggest that by applying 30 mg of PCL, 2% of PVA, 80 ml of continuous phase volume and 0.5 of TMC to QT ratio
2. This ratio was applied to prepare nanosphere at the initial aqueous phase. The results of response surface analysis suggest that by applying 30 mg of PCL, 2% of PVA, 80 ml of continuous phase volume and 0.5 of TMC to QT ratio 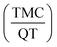 , it is possible to achieve particles with a size of 205 ± 2 nm and drug encapsulation efficiency of 53 ± 2%. Finally, the release of optimal synthesized nanospheres were investigated at the experimental environment with pH = 7.4 and the simulated SGF and SIF conditions. The results reveal a slow and simultaneous release of TMC and QT, which is desirable for drug delivery systems.
, it is possible to achieve particles with a size of 205 ± 2 nm and drug encapsulation efficiency of 53 ± 2%. Finally, the release of optimal synthesized nanospheres were investigated at the experimental environment with pH = 7.4 and the simulated SGF and SIF conditions. The results reveal a slow and simultaneous release of TMC and QT, which is desirable for drug delivery systems.
Notes and references
- S. J. Oh, O. Kim, J. S. Lee, J.-A. Kim, M. R. Kim, H. S. Choi, J.-H. Shim, K. W. Kang and Y. C. Kim, Food Chem. Toxicol., 2010, 48, 3227–3234 CrossRef CAS PubMed.
- L. C. Hartmann, A. C. Degnim, R. J. Santen, W. D. Dupont and K. Ghosh, N. Engl. J. Med., 2015, 372, 78–89 CrossRef PubMed.
- J. Varshosaz, F. Hassanzadeh, H. Sadeghi-Aliabadi, Z. Larian and M. Rostami, Chem. Eng. J., 2014, 240, 133–146 CrossRef CAS.
- Y. S. Elnaggar, M. A. El-Massik and O. Y. Abdallah, Int. J. Pharm., 2009, 380, 133–141 CrossRef CAS PubMed.
- W. Shelly, M. W. Draper, V. Krishnan, M. Wong and R. B. Jaffe, Obstet. Gynecol. Surv., 2008, 63, 163–181 Search PubMed.
- A. Lai, M. Kahraman, S. Govek, J. Nagasawa, C. Bonnefous, J. Julien, K. Douglas, J. Sensintaffar, N. Lu and K.-j. Lee, J. Med. Chem., 2015, 58, 4888–4904 CrossRef CAS PubMed.
- C. K. Osborne, E. B. Coronado-Heinsohn, S. G. Hilsenbeck, B. L. McCue, A. E. Wakeling, R. A. McCleland, D. L. Manning and R. I. Nicholson, J. Natl. Cancer Inst., 1995, 87, 746–750 CrossRef CAS PubMed.
- A. K. Jain, K. Thanki and S. Jain, Mol. Pharmaceutics, 2013, 10, 3459–3474 CrossRef CAS PubMed.
- B. Fisher, J. P. Costantino, C. K. Redmond, E. R. Fisher, D. L. Wickerham and W. M. Cronin, J. Natl. Cancer Inst., 1994, 86, 527–537 CrossRef CAS PubMed.
- F. H. Epstein, M. E. Mendelsohn and R. H. Karas, N. Engl. J. Med., 1999, 340, 1801–1811 CrossRef PubMed.
- E. Coezy, J.-L. Borgna and H. Rochefort, Cancer Res., 1982, 42, 317–323 CAS.
- J. Cuzick, I. Sestak, S. Cawthorn, H. Hamed, K. Holli, A. Howell, J. F. Forbes and I.-I. Investigators, Lancet Oncol., 2015, 16, 67–75 CrossRef CAS PubMed.
- R. R. Love, R. B. Mazess, H. S. Barden, S. Epstein, P. A. Newcomb, V. C. Jordan, P. P. Carbone and D. L. DeMets, N. Engl. J. Med., 1992, 326, 852–856 CrossRef CAS PubMed.
- A. Kumari, S. K. Yadav and S. C. Yadav, Colloids Surf., B, 2010, 75, 1–18 CrossRef CAS PubMed.
- S. Yang, P. Zong, J. Hu, G. Sheng, Q. Wang and X. Wang, Chem. Eng. J., 2013, 214, 376–385 CrossRef CAS.
- Y. Sun, L. Du, Y. Liu, X. Li, M. Li, Y. Jin and X. Qian, Int. J. Pharm., 2014, 469, 31–39 CrossRef CAS PubMed.
- J. Shukla, U. Sharma, R. Kar, I. K. Varma, S. Juyal, N. R. Jagannathan and G. P. Bandopadhyaya, Nanomedicine, 2009, 4, 895–902 CrossRef CAS PubMed.
- D. S. Shaker, M. K. Ghorab, A. Klingner and M. S. Teiama, Int. J. Pharm. Pharm. Sci., 2013, 5, 429–437 CAS.
- W. Ronde and F. H. Jong, Reprod. Biol. Endocrinol., 2011, 9, 93 CrossRef PubMed.
- O. Coskun, M. Kanter, A. Korkmaz and S. Oter, Pharmacol. Res., 2005, 51, 117–123 CrossRef CAS PubMed.
- H. S. Lin, S. Y. Chan, K. S. Y. Low, M. L. Shoon and P. C. Ho, J. Pharm. Sci., 2000, 89, 260–267 CrossRef CAS PubMed.
- S. Caltagirone, F. O. Ranelletti, A. Rinelli, N. Maggiano, A. Colasante, P. Musiani, F. B. Aiello and M. Piantelli, Am. J. Respir. Cell Mol. Biol., 1997, 17, 51–59 CrossRef CAS PubMed.
- M. Paulpandi, K. Kavithaa, S. Sumathi and P. Padma, Ann. Oncol., 2013, 24, iii20 CrossRef.
- R. Diab, C. Jaafar-Maalej, H. Fessi and P. Maincent, AAPS J., 2012, 14, 688–702 CrossRef CAS PubMed.
- X. Wang, Y. Wang, K. Wei, N. Zhao, S. Zhang and J. Chen, J. Mater. Process. Technol., 2009, 209, 348–354 CrossRef CAS.
- V. Sinha, K. Bansal, R. Kaushik, R. Kumria and A. Trehan, Int. J. Pharm., 2004, 278, 1–23 CrossRef CAS PubMed.
- V. Natarajan, N. Krithica, B. Madhan and P. K. Sehgal, J. Pharm. Sci., 2011, 100, 195–205 CrossRef CAS PubMed.
- D. Ibraheem, M. Iqbal, G. Agusti, H. Fessi and A. Elaissari, Colloids Surf., A, 2014, 445, 79–91 CrossRef CAS.
- M. E. Brewster, R. Vandecruys, J. Peeters, P. Neeskens, G. Verreck and T. Loftsson, Eur. J. Pharm. Sci., 2008, 34, 94–103 CrossRef CAS PubMed.
- Y.-Y. Yang, T.-S. Chung, X.-L. Bai and W.-K. Chan, Chem. Eng. Sci., 2000, 55, 2223–2236 CrossRef CAS.
- W.-T. Wang, R. Chen, J.-H. Xu, Y.-D. Wang and G.-S. Luo, Chem. Eng. J., 2015, 263, 412–418 CrossRef CAS.
- J. Tukker, M. Blankenstein and J. Nortier, J. Pharm. Pharmacol., 1986, 38, 888–892 CrossRef CAS PubMed.
- Y. He, Z. He, F. He and H. Wan, Pharmacogn. Mag., 2012, 8, 263 CrossRef CAS PubMed.
- J. M. Colwell, Synthesis of Polycaprolactone Polymers for Bone Tissue Repair, PhD thesis, Queensland University of Technology, 2008 Search PubMed.
- P. P. Muzumdar, C. U. o. T. S. o. Pharmacy, Curtin University of Technology, 1999, p. 35 Search PubMed.
- T. Radhakrishna, A. Narasaraju, M. Ramakrishna and A. Satyanarayana, J. Pharm. Biomed. Anal., 2003, 31, 359–368 CrossRef CAS PubMed.
- M. I. Toral, S. Pope, S. Quintanilla and P. Richter, Int. J. Pharm., 2002, 249, 117–126 CrossRef.
- C. B. Woitiski, F. Veiga, A. Ribeiro and R. Neufeld, Eur. J. Pharm. Biopharm., 2009, 73, 25–33 CrossRef CAS PubMed.
- J.-C. Jeong, J. Lee and K. Cho, J. Controlled Release, 2003, 92, 249–258 CrossRef CAS PubMed.
- I. El-Gibaly, Int. J. Pharm., 2002, 232, 199–211 CrossRef CAS PubMed.
- J. P. Rao and K. E. Geckeler, Prog. Polym. Sci., 2011, 36, 887–913 CrossRef CAS.
- Y.-Y. Yang, T.-S. Chung and N. P. Ng, Biomaterials, 2001, 22, 231–241 CrossRef CAS PubMed.
- M. Li, O. Rouaud and D. Poncelet, Int. J. Pharm., 2008, 363, 26–39 CrossRef CAS PubMed.
- C. Rouzes, M. Leonard, A. Durand and E. Dellacherie, Colloids Surf., B, 2003, 32, 125–135 CrossRef CAS.
- V. Natarajan, P. Saravanakumar and B. Madhan, Int. J. Biol. Macromol., 2012, 50, 1091–1094 CrossRef CAS PubMed.
- B. Surnar and M. Jayakannan, Biomacromolecules, 2013, 14, 4377–4387 CrossRef CAS PubMed.
- G. Tiwari, Int. J. Pharma Bio Sci., 2010, 48 Search PubMed.
- M. E. Davis and M. E. Brewster, Nat. Rev. Drug Discovery, 2004, 3, 1023–1035 CrossRef CAS PubMed.
- J. S. Chawla and M. M. Amiji, Int. J. Pharm., 2002, 249, 127–138 CrossRef CAS PubMed.
- I. Mircioiu, V. Anuta, N. Ibrahim and C. Mircioiu, Farmacia, 2012, 60, 315–324 CAS.
- S. Abbasi, A. Paul, W. Shao and S. Prakash, J. Drug Delivery, 2012, 686108 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21166f |
| This journal is © The Royal Society of Chemistry 2016 |