Enhanced ethanol gas sensing performance of zinc–tin–vanadium oxide nanocomposites at room temperature
Abstract
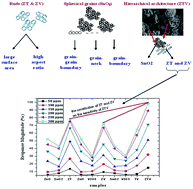
A zinc–tin–vanadium oxide (ZTV) nanocomposite is synthesised via a hydrothermal route followed by calcination, characterised by various state-of-the-art techniques and tested for ethanol sensing behaviour (0–300 ppm) at room temperature. The synergistic effect made ZTV a unique ethanol sensor (98.96%) with a fast adsorption and desorption rate of 32 s and 6 s, respectively. The morphological contribution from the zinc–tin oxide nanocomposite (ZT) and zinc–vanadium oxide nanocomposite (ZV) in the ZTV system provides a larger surface area which in turn promotes a higher number of surface active sites for the adsorption of ethanol molecules on the surface. The catalytic activity along with different reductive–oxidative states had a larger impact on the enhanced ethanol sensing ability of the ZTV system even at room temperature. In this present work, the novel material, ZTV which exhibits excellent ethanol sensing characteristics at room temperature is investigated and the mechanism behind the sensing behaviour of ZTV is elucidated based on its structure and morphology.

 Please wait while we load your content...
Please wait while we load your content...