Antibiotic adjuvants from Buxus sempervirens to promote effective treatment of drug-resistant Staphylococcus aureus biofilms†
A. C. Abreua,
D. Paulet b,
A. Coqueirob,
J. Malheiro
b,
A. Coqueirob,
J. Malheiro a,
A. Borgesa,
M. J. Saavedrac,
Y. H. Choi*b and
M. Simões
a,
A. Borgesa,
M. J. Saavedrac,
Y. H. Choi*b and
M. Simões *a
*a
aLEPABE, Department of Chemical Engineering, Faculty of Engineering, University of Porto, Rua Dr. Roberto Frias, s/n, 4200-465 Porto, Portugal. E-mail: mvs@fe.up.pt; Tel: +351 225081654
bNatural Products Laboratory, Institute of Biology, Leiden University, Leiden, The Netherlands. E-mail: y.choi@chem.leidenuniv.nl; Tel: +31 715274510
cCECAV, Veterinary and Animal Science Research Center and Veterinary Science Department, University of Trás-os-Montes and Alto Douro, Apartado 1013, 5000-801 Vila Real, Portugal
First published on 29th September 2016
Abstract
Plants have been long scrutinized in the quest for new antibiotics, but no strong antibiotic molecule was ever found. Evidence exists that most phytochemicals have a regulatory or adjuvant effect on other antibacterial compounds, thus promoting a greater therapeutic effect. The current study assessed twenty-eight plants from different families for their antibacterial activity and as adjuvants in antibiotic therapy against Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA). Eucalyptus globulus, Castanea sativa, Agrimonia eupatoria and Fraxinus excelsior methanolic extracts showed antibacterial activity with minimal inhibitory concentrations (MICs) of 0.125–0.5, 0.5–1.0, 1.0–2.0, and 2.0–4.0 g L−1, respectively. Non-antibacterial plants were assessed in combination with ampicillin, oxacillin, ciprofloxacin, erythromycin and tetracycline by a modified disc diffusion test. Methanolic extracts of Acacia dealbata, Prunus spp. plants, Centaurea nigra, Eupatorium cannabium and Buxus sempervirens showed a potentiating effect mostly of ciprofloxacin, erythromycin and tetracycline. B. sempervirens was selected for its potentiating activity and applied against S. aureus biofilms. B. sempervirens (1 g L−1) was able to cause an 88% reduction of S. aureus within 1 h exposure. Further phytochemical investigation of B. sempervirens allowed to identify betulinic acid as a major component, together with other triterpenoids. Betulinic acid and other common terpernoids – lupeol, betulin, hederagenin, ursolic acid and oleanolic acid, were tested for antibacterial and antibiotic-potentiating activities. Among the tested compounds, oleanolic acid and ursolic acid – were highlighted, showing MIC of 62.5 and 15.6 mg L−1, respectively, against S. aureus. Additionally, oleanolic acid showed synergism when combined with tetracycline and erythromycin and caused biofilm reductions of 70, 81 and 85% when applied at 1/2 MIC, MIC and 2 × MIC, respectively.
Introduction
Two major circumstances have accentuated the quest for new antibacterial agents and alternative therapies in the last decades. Primarily, because microbes, due to their incredible and innate adaptability, seem to have at least equal chances for survival as scientists and pharmaceutical industries develop methods to kill them.3 Multidrug-resistant (MDR) bacteria are responsible for a large number of nosocomial but also community-acquired infections and are spreading all over the globe.4 Additionally, the limitation of our current arsenal of effective antibiotics accompanied by the lack of new antimicrobial alternatives are prompting the beginning of the “post-antibiotic era”, which threats all the achievements of modern medicine.Since the beginning of mankind plants were undoubtedly the most important source of therapeutic remedies with an enormous range of applications. The earliest records of natural products were depicted from Mesopotamia (2600 B.C.) and included oils from cypress (Cupressus sempervirens) and myrrh (Commiphora species), which are still used today to treat coughs, colds and inflammation.5 Many plant extracts and their phytochemical constituents are known to have antimicrobial activities.6 However, it can be rapidly established that this effort of finding individual active antibiotics in plants has been difficult, since the spectrum of activity of purified components is often non-specific (thus toxic) or very narrow, and for sure weaker than compounds from other sources such as fungi and bacteria.7 However, plants can still fight most of their infections successfully, which proves that plant defence mechanisms are still not well understood.
Plants have faced most of their natural enemies for millions of years which allowed them to co-evolve and learn how to survive to their attacks.8 In fact, they do not produce single strong antibacterial compounds as their main defence mechanism, but hundreds of structurally different chemicals with a wide range of activity.4 Some of them are antimicrobial and act synergistically between each other to produce an enhanced effect against the pathogen. Others are non-antimicrobials, but can improve solubility, absorption and stability of the active compounds. At last but not least, some phytochemicals have been associated with an antibiotic adjuvant activity, especially due to the inhibition of the resistance mechanisms from plant pathogens. Efflux pump inhibitors (EPI) produced by plants have been extensively found and reported,9–12 as well as inhibitors of PBP2a; such as baicalein, tellimagrandin I, rugosin B and corilagin,9,13,14 among others. The inhibition of the pathogen resistance mechanisms is a strategy already implemented in clinic. Clavulanic acid, which inhibits β-lactamases despite its weak antibacterial activity, combined with amoxicillin has proven to be remarkably effective in controlling a wide range of bacterial infections for two decades.15 Plants offer an untapped source of such adjuvant compounds. The aim of this study was first to evaluate the ability of a considerable range of different plants belonging to different families (in order to generate chemical variation) for their antibacterial activity against S. aureus strains, including efflux pump overexpressing and MRSA strains. The plants showing no detectable antibacterial activity were then assessed for their antibiotic-potentiation ability with five antibiotics. The antibiotics chosen (ampicillin and oxacillin – β-lactam, ciprofloxacin – fluoroquinolone, erythromycin – macrolide, and tetracycline) have more limited application nowadays due to increased bacterial tolerance. Additionally, since many reports have shown that staphylococcal infections were associated with biofilm formation, the biofilm control activity promoted by one promising plant extract, which was highlighted among the plants species selected, was evaluated as well.
Materials and methods
Plant material
Twenty-eight Portuguese medicinal, invasive and fruit plant species were mostly collected in the region of Trás-os-Montes and Beira Transmontana (Portugal) and characterized by the Botanical Garden from Vila Real (Portugal), (Table 1). The leaves of all plants were harvested, separated and immediately frozen in liquid N2 in order to avoid unwanted enzymatic reactions and stored at −20 °C until analysis.| Plant name | Common name | Family | Class.* | |
|---|---|---|---|---|
| a (—) Medicinal use not known (for the plant leaves), (*) classification according to the ethnopharmacological relevance described by as: 1 = very minor uses, 2 = reasonably useful plants, 3 = could be grown as standard crops, 4 = very useful plants, 5 = great value. PFAF (2016).1 | ||||
| 1 | Acacia dealbata | Mimosa | Fabaceae | — |
| 2 | Genista tridentata | Carqueja | Fabaceae | — |
| 3 | Prunus domestica | Plum | Rosaceae | 2 |
| 4 | Prunus avium | Wild cherry | Rosaceae | 2 |
| 5 | Prunus persica | Peach tree | Rosaceae | 3 |
| 6 | Pyrus communis | Pear tree | Rosaceae | 1 |
| 7 | Agrimonia eupatoria | Church steeples | Rosaceae | 3 |
| 8 | Eriobotrya japonica | Loquat | Rosaceae | 3 |
| 9 | Crataegus monogyna | Hawthorn | Rosaceae | 5 |
| 10 | Rubus idaeus | Wild raspberry | Rosaceae | 3 |
| 11 | Malus communis | Apple tree | Rosaceae | 2 |
| 12 | Eupatorium cannabinum | Hemp agrimony | Asteraceae | 3 |
| 13 | Centaurea nigra | Black knapweed | Asteraceae | 2 |
| 14 | Physalis angulata | Cutleaf ground-cherry | Solanaceae | 1 |
| 15 | Cyphomandra betacea | Tree tomato | Solanaceae | — |
| 16 | Nerium oleander | Oleander | Apocynaceae | 2 |
| 17 | Trachelospermum jasminoides | Star jasmine | Apocynaceae | 2 |
| 18 | Eucalyptus globulus | Blue gum tree | Myrtaceae | 4 |
| 19 | Calluna vulgaris | Calluna | Ericaceae | 2 |
| 20 | Ficus carica | Fig tree | Moraceae | 2 |
| 21 | Castanea sativa | Sweet chestnut | Fagaceae | 2 |
| 22 | Juglans regia | Walnut | Juglanduceae | 3 |
| 23 | Diospyros kaki | Japanese persimmon | Ebenaceae | 3 |
| 24 | Vitis vinifera | Grape vine | Vitaceae | 2 |
| 25 | Fraxinus excelsior | European ash | Oleaceae | 2 |
| 26 | Actinidia chinensis | Chinese gooseberry | Actinidiaceae | 2 |
| 27 | Buxus sempervirens | Common box | Buxaceae | 2 |
| 28 | Pteridium aquilinum | Bracken | Polypodiaceae | 2 |
Plant samples preparation and extraction procedure
The plant materials (5 g) were freeze-dried and extracted with 50 mL of MeOH at 30 °C, stirring at 150 rpm for 60 min. The samples were filtrated and re-extracted with 50 mL MeOH for 60 min. The resulting extracts were combined and the solvent was evaporated at low temperature (<40 °C) under reduced pressure. The dried MeOH extracts were stored at −20 °C.Bacterial strains
Five S. aureus strains were included in this study: the collection strain S. aureus CECT 976, used as the model microorganism for antimicrobial tests with phytochemical compounds;16,17 S. aureus SA1199B, which overexpresses the NorA MDR efflux pump; S. aureus RN4220, which contains plasmid pU5054 (that carries the gene encoding the MsrA macrolide efflux protein); S. aureus XU212, which possesses the TetK efflux pump; and the clinical MRSA strain MJMC001, which was obtained from the Hospital Centre of Trás-os-Montes and Alto Douro, Vila Real (Portugal). Prior to use, each strain that had been kept at −80 °C was transferred onto Mueller–Hinton (MH, Merck Milllipore, Germany) agar plate, grown overnight, and inoculated into MH broth at 37 °C and under agitation (150 rpm).Antibiotics and other chemicals
The antibiotics: ampicillin, ciprofloxacin, erythromycin, oxacillin and tetracycline, were obtained from Sigma-Aldrich (St. Louis, Missouri, EUA) and prepared according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.2 Betulinic acid, lupeol, betulin, hederagenin, ursolic acid and oleanolic acid were purchased from Sigma-Aldrich and their stock solutions (1 g L−1) were prepared in dimethyl sulfoxide (DMSO).Antibacterial susceptibility testing
Before testing, the dried MeOH extracts were prepared in DMSO. The MIC of each plant extract was determined by microdilution technique according to the CLSI guidelines.2 Bacteria (∼106 CFU mL−1) were inoculated into MH broth and 200 μL per well were dispensed in 96-well microtiter plates, along with 2-fold dilutions of the MeOH extracts. The highest concentration tested for each plant extract was 4 g L−1. Plant extracts did not exceed 5% (v/v) of the volume of the well. MIC was defined as the lowest concentration of the extract that inhibited bacterial growth after 24 h of incubation at 37 °C. The bacterial growth was determined at 600 nm using a microplate reader (Spectramax M2e, Molecular Devices, Inc., Sunnyvale, USA). The highest concentration of DMSO remaining after dilution (5% (v/v)) caused no inhibition of bacterial growth (data not shown). Three independent experiments were performed for each plant extract.Antibiotic-potentiation testing – disc diffusion test
Disc diffusion test is the most suitable technique for plant extracts, since allows adequate visualization and detection of the potentiating effects as evidenced in diverse studies.16,17 Plant extracts showing no MIC below 4 g L−1 were tested for an antibiotic-potentiating activity at several concentrations (between 0.125 to 4 g L−1), in order to define the minimal/optimal concentration causing antibiotic potentiation. Each extract prepared in DMSO was added to MH agar (after autoclaved and cooled) yielding the final concentration desired. Plant extracts did not exceed 5% (v/v) of the total volume of medium. Then 20 mL of medium was poured into 90 mm Petri dishes. The bacterial suspensions were adjusted to 0.5 McFarland standards and seeded over hardened MH agar Petri dishes using a sterilized cotton swab. Sterile blank discs (6 mm diameter; Oxoid) were placed on the agar plates. Ten μL of each antibiotic prepared according to the CLSI guidelines2 (ampicillin – 10 μg per disc; ciprofloxacin – 5 μg per disc; erythromycin – 15 μg per disc; tetracycline – 30 μg per disc; and oxacillin – 1 μg per disc) was added to the blank discs. Discs with antibiotics were also applied on plant-free MH agar plates, as well as discs with DMSO (negative control). No inhibition zone was obtained with DMSO (data not shown). The plates were incubated at 37 °C for 24 h. After incubation, all the inhibition zone diameters (IZD) were recorded. On simple MH plates, antibiotic IZDs were analysed and strains were characterized for their susceptibility/resistance profile to each antibiotic according to CLSI guidelines.2 The IZDs obtained in the plates containing the plant extract (IZDantib.+plant) were compared to the single antibiotic IZDs (IZDantib.). The antibiotic-potentiating activity of each plant extract was categorized into three classes: indifferent/no potentiation (+): (IZDantib.+plant − IZDantib.) < 4 mm; low potentiation/additive effect (++): 4 ≤ (IZDantib.+plant − IZDantib.) < 6 mm; and potentiation effect (+++): (IZDantib.+plant − IZDantib.) ≥ 6 mm of inhibition of growth of S. aureus.16,17 All tests were performed in triplicate in three independent experiments.Antibiotic-potentiation testing – checkerboard
The checkerboard assay was performed in order to assess the synergy between antibiotics and those phytochemicals with antibacterial activity. This method was performed according to CLSI guidelines18 as described in previous studies.16,17 Both compounds yielded final concentrations between 2 × MIC to 1/64 × MIC. Combinations did not exceed 5% (v/v) of the volume used in each well (200 μL). Growth controls consisted in 5% (v/v) DMSO. Incubation was performed for 24 h at 37 °C and readings were determined spectrophotometrically at 600 nm. All MIC determinations were performed in triplicate. Antibiotic (A) + phytochemical (B) interactions were classified using the fractional inhibitory concentration (FIC) index: FICI = FIC(A) + FIC(B), where FIC(A) is the ratio between the MIC of drug A in combination and the MIC of drug A alone and FIC(B) is the ratio of the MIC of drug B in combination and the MIC of drug B alone.19 A FICI value of ≤0.5 was interpreted as synergy, >4 as antagonism and >0.5–4 as indifferent.Biofilm control experiment
B. sempervirens was chosen for its promising antibiotic-potentiating activity and evaluated for its ability to control (remove and inactivate) biofilms of S. aureus CECT 976 within 1 h exposure. Biofilms were developed according to a modified microtiter plate test as described previously.20 Overnight cultures (∼106 cells per mL) were added to sterile 96-well polystyrene microtiter plates (Orange Scientific, USA) to form biofilms at 37 °C and stirring at 150 rpm for 24 h. Afterwards, the medium was removed and the biofilms were exposed to the antibiotics and to the plant extract individually and in combination at 37 °C and stirring at 150 rpm for 1 h. Antibiotic solutions were applied at MIC and 50 × MIC against biofilms. The MeOH extract of B. sempervirens was applied at concentrations ranging from 0.05 to 5 g L−1. Drug combinations did not exceed 5% (v/v) of the well (200 μL). After incubation, biofilms were washed twice with saline solution (0.85% NaCl), scrapped and diluted for colony forming units (CFU) counting. The numbers of CFU per unit of adhesion area (CFU cm−2) were assessed in MH agar. Reduction (%) of the number of CFU cm−2 (compared with DMSO growth control) was also assessed.Fractionation of active extract of Buxus sempervirens
B. sempervirens leaves (160 g) were extracted with 500 mL of MeOH following the process described above. The MeOH extract was taken to dryness and redissolved in 225 mL of H2O/MeOH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and successively partitioned with 3 × 112.5 mL of CHCl2 and n-BuOH, respectively. All the fractions were analyzed by 1H NMR and tested for both antibacterial and antibiotic-potentiating activities. Afterwards, the n-BuOH fraction (1.2 g) was further submitted to phytochemical investigation. The n-BuOH fraction was subjected to medium pressure liquid chromatography (MPLC, Sepacore, Büchi, Switzerland) in a silica gel column (80 g, 200 × 35 mm, i.d., Büchi), eluted with a gradient of CHCl3 (A): MeOH + HOAc 2% (B) as follow: 10% B for 15 min; linear increase 10–30% B in 5 min; isocratic elution using 30% B for 20 min; linear increase 30–50% B in 5 min, and finally 50% B for 10 min. The flow rate was 20 mL min−1 and the analysis was monitored by UV spectrometer at 220, 254, 280 and 365 nm. Collection was performed by volume were each fraction contained 20 mL, totalizing 53 fractions. The fractions were analyzed by analytical TLC and combined into 8 subfractions. TLC was performed using silica gel TLC plates (Merck, Darmstadt, Germany) with CHCl3
1) and successively partitioned with 3 × 112.5 mL of CHCl2 and n-BuOH, respectively. All the fractions were analyzed by 1H NMR and tested for both antibacterial and antibiotic-potentiating activities. Afterwards, the n-BuOH fraction (1.2 g) was further submitted to phytochemical investigation. The n-BuOH fraction was subjected to medium pressure liquid chromatography (MPLC, Sepacore, Büchi, Switzerland) in a silica gel column (80 g, 200 × 35 mm, i.d., Büchi), eluted with a gradient of CHCl3 (A): MeOH + HOAc 2% (B) as follow: 10% B for 15 min; linear increase 10–30% B in 5 min; isocratic elution using 30% B for 20 min; linear increase 30–50% B in 5 min, and finally 50% B for 10 min. The flow rate was 20 mL min−1 and the analysis was monitored by UV spectrometer at 220, 254, 280 and 365 nm. Collection was performed by volume were each fraction contained 20 mL, totalizing 53 fractions. The fractions were analyzed by analytical TLC and combined into 8 subfractions. TLC was performed using silica gel TLC plates (Merck, Darmstadt, Germany) with CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH
MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HOAc (7.5
HOAc (7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2). These eight fractions were analysed by 1H NMR and for their antibacterial-potentiating analysis.
0.2). These eight fractions were analysed by 1H NMR and for their antibacterial-potentiating analysis.
NMR analysis
Five hundred microliters of CH3OH-d4 were added to dried samples, and the resultant mixtures were vortexed for 10 s and sonicated for 20 min at 42 kHz, followed by centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at room temperature for 5 min. Three hundred microliters of the supernatant were transferred to a 3 mm micro-NMR tube and analysed. 1H NMR spectra were recorded at 25 °C on a 600 MHz Bruker DMX-600 spectrometer (Bruker, Karlsruhe, Germany) operating at a proton frequency of 600.13 MHz. MeOH-d4 was used as the internal lock. 1H NMR experimental parameters were the following: 128 scans requiring 10 min and 26 s acquisition time, 0.16 Hz per point, pulse width (PW) = 30° (11.3 μs), and relaxation delay (RD) = 1.5 s. FIDs were Fourier transformed with LB = 0.3 Hz. The resulting spectra were manually phased and baseline corrected, and calibrated to residual CH3OH-d4 at 3.3 ppm, using TOPSPIN 3.2 software (Bruker BioSpin GmbH, Rheinstetten, Germany).
000 rpm at room temperature for 5 min. Three hundred microliters of the supernatant were transferred to a 3 mm micro-NMR tube and analysed. 1H NMR spectra were recorded at 25 °C on a 600 MHz Bruker DMX-600 spectrometer (Bruker, Karlsruhe, Germany) operating at a proton frequency of 600.13 MHz. MeOH-d4 was used as the internal lock. 1H NMR experimental parameters were the following: 128 scans requiring 10 min and 26 s acquisition time, 0.16 Hz per point, pulse width (PW) = 30° (11.3 μs), and relaxation delay (RD) = 1.5 s. FIDs were Fourier transformed with LB = 0.3 Hz. The resulting spectra were manually phased and baseline corrected, and calibrated to residual CH3OH-d4 at 3.3 ppm, using TOPSPIN 3.2 software (Bruker BioSpin GmbH, Rheinstetten, Germany).
Statistical analysis
For statistical analysis, the in vitro results were analysed by Student's t test using the statistical program SPSS version 19.0 (Statistical Package for the Social Sciences). Statistical calculations were based on a confidence level ≥95% (P < 0.05) which was considered statistically significant.Results and discussion
Regardless of their medicinal uses, all plants have their own defence mechanisms from pathogens, producing a wide range of different chemicals for that purpose. In this study, twenty-eight plants were assessed for their activity for potentiating antibiotics against diverse S. aureus strains. The plants were selected among different families in order to be able to test a large variety of extracts and metabolites. Table 1 describes the plants tested in this study as well as their ethnopharmacological relevance. The MIC for all MeOH extracts was determined for concentrations below 4 g L−1. Only four plant extracts showed a detectable MIC (Table 2). Eucalyptus globulus presented the highest antibacterial activity with a MIC between 0.125 and 0.25 g L−1 against the diverse S. aureus strains, including MRSA. This activity is in accordance with its therapeutic use.1 Other studies reported that the essential oils from the leaves and the flowers of E. globulus inhibited the growth of Escherichia coli, S. aureus,21 MRSA and vancomycin-resistant enterococci (VRE) Enterococcus faecalis.22 Concerning the other antibacterial plants, previous studies corroborate the results obtained: Basile, et al.23 found MIC in the range of 64–0.256 g L−1 of the aqueous extract of Castanea sativa (pH 3.0) against several bacteria including S. aureus; Agrimonia eupatoria was reported for its inhibitory effects against S. aureus;24–26 other authors reported antibacterial activity of n-hexane and CHCl2 extracts of Fraxinus excelsior against S. aureus (MIC of 0.125 and 0.25 g L−1, respectively) and MRSA strains (MIC of 0.5 and 1.0 g L−1, respectively) Middleton, et al.27| Plant | MIC (g L−1) |
|---|---|
| Eucalyptus globulus | 0.125–0.5 |
| Castanea sativa | 0.5–1.0 |
| Agrimonia eupatoria | 1.0–2.0 |
| Fraxinus excelsior | 2.0–4.0 |
The plant extracts that did not show any detectable antibacterial activity were further evaluated for antibiotic-potentiating activity with five antibiotics by the disc diffusion method. First, the classification of S. aureus strains according to their resistance profile was performed based on the comparison of the MICs/IZDs results and the susceptibility breakpoints of CLSI guidelines,2 as shown in Table 3. Table 4 shows the antibiotic-potentiation results obtained for each plant extract. Only plant extracts showing potentiation of at least one antibiotic were included. No IZD originated by the combinations between plant extracts and antibiotics was ever inferior to that promoted by the antibiotic alone (P > 0.05). Plants promoting antibiotic-potentiation were: plants from Rosaceae family, including all Prunus spp. and Pyrus communis, Acacia dealbata, both Asteraceae plants, Centaurea nigra and Eupatorium cannabium, as well as Buxus sempervirens.
| CECT 976 | SA1199Ba | XU212a | RN4220a | MJMC001 | ||
|---|---|---|---|---|---|---|
| a Strains SA1199B, XU212 and RN4220 were only exposed to the antibiotic they are resistant to: CIP, TET and ERY, respectively.b IZD: inhibition zone diameter; AMP: ampicillin; OXA: oxacillin; CIP: ciprofloxacin, TET: tetracycline; ERY: erythromycin. | ||||||
| AMP | IZD | 36.0 ± 1.0 | 0.0 ± 0.0 | |||
| MIC | 1.5 | — | — | — | 64 | |
| Class. | S | R | ||||
| OXA | IZD | 39.7 ± 0.6 | 0.0 ± 0.0 | |||
| MIC | 0.48 | — | — | — | 128 | |
| Class. | S | R | ||||
| CIP | IZD | 33.3 ± 0.6 | 13.0 ± 0.0 | 0.0 ± 0.0 | ||
| MIC | 1 | 4 | — | — | 256 | |
| Class. | S | R | R | |||
| TET | IZD | 23.7 ± 0.6 | 9.0 ± 1.0 | 26.0 ± 0.0 | ||
| MIC | 0.96 | — | 32 | — | 0.5 | |
| Class. | S | R | S | |||
| ERY | IZD | 26.3 ± 0.6 | 0.0 ± 0.0 | 12.5 ± 0.6 | ||
| MIC | 0.24 | — | — | 256 | 96 | |
| Class. | S | R | R | |||
| Plant | CECT 976 | SA1199Ba | XU212a | RN4220a | MJMC001 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g L−1 | AMP | OXA | CIP | TET | ERY | CIP | TET | ERY | AMP | OXA | CIP | TET | ERY | |
| a Strains SA1199B, XU212 and RN4220 were only exposed to the antibiotic they are resistant to: CIP, TET and ERY, respectively.b Indifference (+): (IZDantib.+plant − IZDantib.) < 4 mm; additive effect (++): 4 ≤ (IZDantib.+plant − IZDantib.) < 6 mm; potentiation (+++): (IZDantib.+plant − IZDantib.) ≥ 6 mm of inhibition of S. aureus growth. AMP: ampicillin; OXA: oxacillin; CIP: ciprofloxacin, TET: tetracycline; ERY: erythromycin; IZD: inhibition zone diameter. | ||||||||||||||
| Acacia dealbata | 2 | + | + | +++ | + | +++ | +++ | + | ++ | + | + | + | + | ++ |
| Pyrus communis | 4 | + | + | + | +++ | +++ | + | ++ | + | + | + | + | + | + |
| Prunus avium | 4 | + | + | + | +++ | +++ | +++ | ++ | +++ | + | + | + | + | ++ |
| Prunus domestica | 4 | + | + | + | +++ | +++ | +++ | ++ | +++ | + | + | + | + | ++ |
| Prunus persica | 4 | + | + | ++ | +++ | ++ | +++ | ++ | +++ | + | + | ++ | + | ++ |
| Centaurea nigra | 1 | + | + | +++ | + | +++ | +++ | + | + | + | + | + | + | + |
| Eupatorium cannabinum | 1 | + | + | + | +++ | +++ | + | + | +++ | + | + | + | + | +++ |
| Ficus carica | 2 | + | + | + | ++ | + | +++ | + | + | + | + | + | + | + |
| Buxus sempervirens | 1 | ++ | ++ | +++ | +++ | +++ | +++ | + | +++ | + | + | + | + | +++ |
Prunus spp. MeOH extracts showed interesting potentiating results though only at high concentrations (4 g L−1). Results were very similar between the three plant extracts. Potentiation/additive effects were mainly found with ciprofloxacin against SA1199B strain, tetracycline against CECT 976 and erythromycin against CECT 976, RN4220 and MJMC001. No study about antibiotic-potentiating activity of these Prunus species was previously reported. The MeOH leaf extracts from A. dealbata (at 2 g L−1) potentiated ciprofloxacin against S. aureus CECT 976 and SA1199B and erythromycin against strains CECT 976, RN4220 and MJMC001 (only additive interactions were obtained against these last two strains). Taguri, et al.28 found generally weak activity of A. dealbata extract against many different bacteria while Olajuyigbe and Afolayan29 found synergistic interactions between Acacia mearnsii and erythromycin, metronidazole, amoxicillin, chloramphenicol and kanamycin against S. aureus. Other MeOH extracts showed significant activities: C. nigra (at 1 g L−1) potentiated ciprofloxacin against CECT 976 and SA1199B and erythromycin against CECT 976; E. cannabium (at 1 g L−1) potentiated tetracycline against strain CECT 976, and erythromycin against CECT 976, RN4220 and MJMC001; P. communis (at 4 g L−1) potentiated tetracycline and erythromycin against CECT 976; and F. carica (at 2 g L−1) potentiated ciprofloxacin against SA1199B. B sempervirens (at 0.5 g L−1) promoted several additive and potentiating effects when combined with all antibiotics against CECT 976; with ciprofloxacin against SA1199B and erythromycin against strains RN4220 and MJMC001.
Ciprofloxacin, erythromycin and tetracycline were potentiated by the plant extracts mentioned even against MRSA. Resistance to these antibiotics can be easily achieved with the expression of efflux pumps from pathogens.30 Most of these plant extracts may be causing efflux pump inhibition in S. aureus, thus explaining the potentiation of ciprofloxacin, tetracycline and erythromycin. Indeed, the number of plant extracts producing efflux pump inhibitors seems to be considerable, as it is being extensively reported.9,10,31,32 No plant extract significantly potentiated β-lactam antibiotics, but additive effects were found with B. sempervirens. Therefore, B. sempervirens seems to act as a general potentiator for the several antibiotics, regardless the antibiotic class. Thus, it is possible that a mechanism, not efflux pump related, is inhibited by B. sempervirens extract.
Antibiotic potentiation can be reached by compounds that are interfering with other mechanisms of the bacterial cell that not involve drug resistance mechanisms, such as quorum-sensing, virulence activation, biofilm formation, adherence to the host tissues,33 etc. Bacterial biofilms are particularly problematic since they become even more resistant to most available antibiotics. Any effective strategy able to impair biofilm formation or disturb, weaken or disperse its structure is urgently needed and for long desired. The MeOH extract of B. sempervirens was analyzed for its ability to control biofilms of the susceptible strain S. aureus CECT 976, against which the antibiotic combinations with B. sempervirens extract were generally more effective.
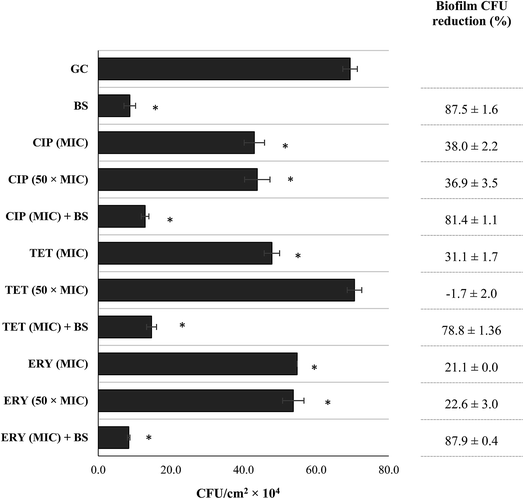
The MeOH extract of B. sempervirens was evaluated against CECT 976 24 h-old biofilms at several concentrations (Fig. 1). It is possible to observe an overall concentration-dependent effect, and increasing concentrations of B. sempervirens extract caused high biofilm removal (P < 0.05), except for 0.25 g L−1 and 5 g L−1, which did not show improvement over the preceding concentrations of 0.1 and 1 g L−1, respectively (P > 0.05). The minimal concentration causing potentiation with planktonic cells (1 g L−1) was the one causing the highest biofilm CFU control (88%). Additionally, combinations between B. sempervirens MeOH extract with ciprofloxacin, tetracycline and erythromycin against 24 h-old biofilms were assessed (Fig. 2). Antibiotics were applied at MIC and 50 × MIC and the extract of B. sempervirens was applied at the concentration causing highest biofilm removal/inactivation (1 g L−1). Concerning the single activity of the antibiotics at MIC, ciprofloxacin, tetracycline and erythromycin promoted a biofilm CFU control of 38, 31 and 21%, respectively. Antibiotic applied at 50 × MIC did not show any improvement over application at MIC for ciprofloxacin and erythromycin (P > 0.05). Tetracycline at 50 × MIC did not cause any biofilm control (similar CFU cm−2 values to the growth control, P > 0.05). This supports the concept of how bacteria are much more protected within a biofilm.34 Comparing individual activities, B. sempervirens MeOH extract surprisingly achieved the best ability to control S. aureus CECT 976 biofilms within 1 h of application, even not showing antibacterial activity at this concentration. This proposes the potential of B. sempervirens extract to disperse biofilms without causing antimicrobial effects. According to Monzón et al.35 it is possible to classify a combination between a plant extract/phytochemical and an antibiotic as synergic if the log10 reduction CFU cm−2 caused by the combination is significantly higher (P < 0.05) than the sum of reductions of individual treatments. In this case, the application of antibiotics at MIC did not promote any significant improvement over activity of the plant extract alone (P > 0.05).
Afterwards, B. sempervirens MeOH extract was submitted to fractionation for the identification of the bioactive compounds. Among the subfractions obtained from the n-BuOH fraction of B. sempervirens, through silica gel column, F1 and F2 were differentiated, showing antibiotic-potentiating activity (at 0.5 g L−1, data not shown). Analysing the 1H NMR for all the eight fractions obtained, it was possible to compare the spectra of the active fractions F1 and F2 with the non-active ones. Bearing in mind the similar activity, some signals were found in both fractions (Fig. 3), which were not found in the non-active ones. The identification was carried out using our in-house library of NMR data of common metabolites. Based on characteristic methyl and olefinic signals it was possible to identify betulinic acid (Fig. 4) as a major component together with oleanane and ursane type terpenoids. Betulinic acid and other similar terpenoids – lupeol, betulin, hederagenin, ursolic acid and oleanolic acid – were tested for their antibacterial activity by microdilution technique as previously explained.
 | ||
| Fig. 3 1H-NMR spectra (0.4–5.0 ppm) of the fractions (F1–F8) obtained from n-BuOH fraction of B. sempervirens; the numbering in the active fractions F1 and F2 is H-number of betulinic acid structure in Fig. 4 are resistant to: CIP, TET and ERY, respectively. | ||
Pentacyclic triterpenoids α-amyrin, betulinic acid and betulinaldehyde, and other related triterpenes such as imberbic acid, oleanolic acid, ursolic acid, ulsolic acid, rotundic acid and zeylasteral have been reported to possess antimicrobial activity against many bacterial species, especially Gram-positive, but also against Gram-negative.36,37 In this study, only oleanolic acid and ursolic acid showed MIC up to 120 mg L−1, which was 62.5 and 15.6 mg L−1, respectively, against S. aureus CECT 976. After MIC determination, these terpenoids were evaluated in combination with antibiotics searching for a synergistic activity through checkerboard method (Table 5). Analysing the FICI values, it is possible to detect synergism only between oleanolic acid with erythromycin and tetracycline (FICI ≤ 0.5) and between ursolic acid and tetracycline (FICI = 0.31) against S. aureus CECT 976. Similarly, Fontanay, et al.38 found MIC for ursolic acid and oleanolic acid of 8 and 32–64 mg L−1 against S. aureus ATCC25923 and ATCC29213 but not for betulinic acid. No MIC was found against one MRSA strain.38 Contrarily, in other study, oleanolic acid was reported to inhibit MRSA with a MIC between 16 and 128 mg L−1 (ref. 39) and to synergize with ampicillin against S. aureus.40 Chung, et al.36 showed that betulinic acid and similar compounds, α-amyrin and betulinaldehyde, inhibited methicillin-susceptible S. aureus (MSSA) and MRSA (MIC between 64 and 512 mg L−1), and synergized with methicillin and vancomycin against the same strains. Therefore, it seems that the antibacterial and synergistic activities of triterpenoids vary widely, not only between susceptibility methods, but also between strains belonging to the same species. Considering the low activity displayed by betulinic acid, which was found in the active fractions of B. sempervirens, other triterpenoids also existing in this plant, as reported for example by Abramson, et al.,41 or other sterols, alkaloids and anthocyanins that are typical of Buxus spp.,42 could synergistically contribute to the antibiotic-potentiation and anti-biofilm effects displayed by this plant. Further isolation of the active fractions of the plant towards the identification of all the involved metabolites is apparently necessary.
| Antibiotic – oleanolic acid | Antibiotic – ursolic acid | |||||
|---|---|---|---|---|---|---|
| MIC fold reduction | MIC fold reduction | FICIa | MIC fold reduction | MIC fold reduction | FICIa | |
| a A FICI value of ≤0.5 was interpreted as synergy, >4 as antagonism and >0.5–4 as indifferent. FIC: fractional inhibitory concentration; FICI: FIC index. AMP: ampicillin; OXA: oxacillin; CIP: ciprofloxacin, TET: tetracycline; ERY: erythromycin. | ||||||
| AMP | 2 | 2 | 1 (I) | 2 | 2 | 1 (I) |
| CIP | 4 | 2 | 0.75 (I) | 8 | 2 | 0.63 (I) |
| TET | 4 | 4 | 0.5 (S) | 4 | 16 | 0.31 (S) |
| ERY | 8 | 4 | 0.38 (S) | 2 | 2 | 1 (I) |
Triterpenoids are widely distributed in the plant kingdom and their therapeutic activities (such as antibacterial, antiviral, antiulcer, anti-inflammatory and anticancer) have been described in numerous reports. Plenty studies were also initiated to identify the cellular targets and molecular mechanisms of triterpenoids. Besides their influence on bacterial gene expression,43 cell autolysis and peptidoglycan turnover,37 oleanolic acid and related compounds also seem to affect the formation and maintenance of biofilms.44 Indeed, terpenes are believed to influence the fatty acid composition of the cell membrane, and thus cell hydrophobicity, which can lead to biofilm eradication.45 To confirm this, oleanolic acid, which caused the best antibiotic-potentiation in this study, was evaluated for its anti-biofilm activity against S. aureus CECT 976 biofilms. Fig. 5 shows the number of CFU cm−2 obtained after 1 h exposure to oleanolic acid at 1/2 MIC, MIC and 2 × MIC as well as with antibiotics at MIC, individually and in combination. Oleanolic acid applied at 1/2 MIC, MIC and 2 × MIC caused high biofilm CFU reduction, 70, 81 and 85%, respectively. The combination between the antibiotics and oleanolic acid never promoted higher biofilm reductions than those obtained with oleanolic acid alone. The exception was the combination of ciprofloxacin (at MIC) with oleanolic acid (at 1/2 MIC), that showed to be significantly than oleanolic acid alone at the same concentration (82%, P < 0.05). However, diverse combinations achieved worst biofilm reductions than those obtained with oleanolic acid alone (P < 0.05): between oleanolic acid and erythromycin, between oleanolic acid (at MIC) and tetracycline (at MIC) and between oleanolic acid (at 2 × MIC) and ciprofloxacin (at MIC).
One could expect that by combining antibiotics with a possible biofilm inhibitor, the outcome would be an improved therapeutic benefit. Nevertheless, probably by applying the combination in a preliminary stage of bacteria adhesion/biofilm formation, the combinations would be more effective, which would explain the potentiation observed. Kurek, et al. (2012)40 also found synergistic antibacterial effects of oleanolic acid in combination with ampicillin against biofilms of S. aureus and S. epidermidis, and with oxacillin against biofilms of L. monocytogenes, S. epidermis and S. aureus. Ursolic acid was found to inhibit biofilm formation of MRSA by reducing amino acids metabolism and expression of adhesins,46 to induce genes related to chemotaxis, mobility and heat shock response, and to repress genes that have functions in cysteine synthesis and sulfur metabolism.47
Conclusions
Restoring the activity of antibiotics that were already accepted and approved for clinical safety aspects, minimal toxicity and side effects, could thereby potentially reduce the costs associated to drug preclinical and clinical development. This strategy is possible by combining antibiotic with adjuvants that are able to inhibit drug-resistance mechanisms expressed by the pathogens. This study allowed to assess the potential of 28 different plant species to be used in co-therapies against S. aureus, a major cause of hospital acquired infections. From the tested plants, four (E. globulus, C. sativa, A. eupatoria and F. excelsior) were found to have antibacterial activity, being in agreement with their traditional uses, and nine (A. dealbata, P. communis, P. avium, P. domestica, P. persica, C. nigra, E. cannabinum, F. carica, B. sempervirens) were able to potentiate antibiotic activity, especially ciprofloxacin, tetracycline and erythromycin. Additionally, this study highlights the potential of B. sempervirens extract, and particularly of triterpenoids for their relevant ability to act against S. aureus biofilms. Nevertheless, besides betulinic acid, the major triterpenoid found in the active fractions of B. sempervirens, other relevant molecules may contribute to the antibiotic-potentiation and anti-biofilm effects exhibited by the plant extract.Acknowledgements
This work was financially supported by: Project POCI-01-0145-FEDER-006939 – Laboratory for Process Engineering, Environment, Biotechnology and Energy – LEPABE funded by FEDER funds through COMPETE2020 – Programa Operacional Competitividade e Internacionalização (POCI) – and by national funds through FCT – Fundação para a Ciência e a Tecnologia; by national funds through FCT – Fundação para a Ciência e a Tecnologia/MEC through the grants of A. C. Abreu (SFRH/BD/84393/2012), J. Malheiro (SFRH/BD/103843/2014) and A. Borges (SFRH/BPD/98684/2013). Dr. A. Coqueiro thanks Ciências sem fronteiras, CAPES Foundation, Ministry of Education of Brazil, for the scholarship.Notes and references
- PFAF, Plants For A Future (PFAF) Database, http://www.pfaf.org/user/default.aspx, accessed May, 2016.
- CLSI, Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing – Twenty-Fourth Informational Supplement, CLSI, Wayne, PA, USA, 2014 Search PubMed.
- J. Berdy, J. Antibiot., 2012, 65, 385–395 CrossRef CAS PubMed.
- R. González-Lamothe, G. Mitchell, M. Gattuso, M. S. Diarra, F. Malouin and K. Bouarab, Int. J. Mol. Sci., 2009, 10, 3400–3419 CrossRef PubMed.
- D. A. Dias, S. Urban and U. Roessner, Metabolites, 2012, 2, 303–336 CrossRef CAS PubMed.
- A. L. A. Siebra, L. R. Oliveira, A. O. B. P. B. Martins, D. C. Siebra, R. S. Albuquerque, I. C. S. Lemos, G. A. Delmondes, S. R. Tintino, F. G. Figueredo, J. G. M. da Costa, H. D. M. Coutinho, I. R. A. Menezes, C. F. B. Felipe and M. R. Kerntopf, Saudi J. Biol. Sci., 2016 DOI:10.1016/j.sjbs.2016.01.019.
- G. Tegos, F. R. Stermitz, O. Lomovskaya and K. Lewis, Antimicrob. Agents Chemother., 2002, 46, 3133–3141 CrossRef CAS PubMed.
- J. P. Anderson, C. A. Gleason, R. C. Foley, P. H. Thrall, J. B. Burdon and K. B. Singh, Funct. Plant Biol., 2010, 37, 499–512 CrossRef PubMed.
- A. C. Abreu, A. J. McBain and M. Simoes, Nat. Prod. Rep., 2012, 29, 1007–1021 RSC.
- M. Stavri, L. J. V. Piddock and S. Gibbons, J. Antimicrob. Chemother., 2007, 59, 1247–1260 CrossRef CAS PubMed.
- E. C. Smith, M. Williamson, N. Wareham, G. Kaatz and S. Gibbons, Phytochemistry, 2007, 68, 210–217 CrossRef CAS PubMed.
- S. Gibbons, M. Oluwatuyi, N. C. Veitch and A. I. Gray, Phytochemistry, 2003, 62, 83–87 CrossRef CAS PubMed.
- M. Shimizu, S. Shiota, T. Mizushima, H. Ito, T. Hatano, T. Yoshida and T. Tsuchiya, Antimicrob. Agents Chemother., 2001, 45, 3198–3201 CrossRef CAS PubMed.
- S. Shiota, M. Shimizu, J. Sugiyama, Y. Morita, T. Mizushima and T. Tsuchiya, Microbiol. Immunol., 2004, 48, 67–73 CrossRef CAS PubMed.
- L. A. Miller, K. Ratnam and D. J. Payne, Curr. Opin. Pharmacol., 2001, 1, 451–458 CrossRef CAS PubMed.
- A. C. Abreu, S. C. Serra, A. Borges, M. J. Saavedra, A. J. McBain, A. J. Salgado and M. Simões, Microb. Drug Resist., 2015, 21, 600–609 CrossRef CAS PubMed.
- A. C. Abreu, S. C. Serra, A. Borges, M. J. Saavedra, A. J. Salgado and M. Simões, Diagn. Microbiol. Infect. Dis., 2014, 79, 125–134 CrossRef CAS PubMed.
- NCCLS/CLSI, National Committee for Clinical Laboratory Standards: Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically – sixth edition: Approved standard M7-A6., NCCLS, Wayne, PA, USA, 2003 Search PubMed.
- M. M. Sopirala, J. E. Mangino, W. A. Gebreyes, B. Biller, T. Bannerman, J.-M. Balada-Llasat and P. Pancholi, Antimicrob. Agents Chemother., 2010, 54, 4678–4683 CrossRef CAS PubMed.
- A. C. Abreu, A. Borges, F. Mergulhão and M. Simões, Int. Biodeterior. Biodegrad., 2014, 86, 34–41 CrossRef CAS.
- R. G. Bachir and M. Benali, Asian Pac. J. Trop. Biomed., 2012, 2, 739–742 CrossRef PubMed.
- D. Srinivasan, S. Nathan, T. Suresh and P. Lakshmana Perumalsamy, J. Ethnopharmacol., 2001, 74, 217–220 CrossRef CAS PubMed.
- A. Basile, S. Sorbo, S. Giordano, L. Ricciardi, S. Ferrara, D. Montesano, R. Castaldo Cobianchi, M. L. Vuotto and L. Ferrara, Fitoterapia, 2000, 71(1), S110–S116 CrossRef CAS PubMed.
- A. Copland, L. Nahar, C. T. M. Tomlinson, V. Hamilton, M. Middleton, Y. Kumarasamy and S. D. Sarker, Fitoterapia, 2003, 74, 133–135 CrossRef CAS PubMed.
- F. Watkins, B. Pendry, A. Sanchez-Medina and O. Corcoran, J. Ethnopharmacol., 2012, 144, 408–415 CrossRef PubMed.
- B. Dulgerm and A. Gonuz, Pak. J. Biol. Sci., 2004, 7, 1559–1562 CrossRef.
- P. Middleton, F. Stewart, S. Al-Qahtani, P. Egan, C. O'Rourke, A. Abdulrahman, M. Byres, M. Middleton, Y. Kumarasamy, M. Shoeba, N. Lutfun, A. Delazar and S. Dey Sarker, Iran. J. Pharm. Res., 2005, 2, 81–86 Search PubMed.
- T. Taguri, T. Tanaka and I. Kouno, Biol. Pharm. Bull., 2006, 29, 2226–2235 CAS.
- O. O. Olajuyigbe and A. J. Afolayan, Int. J. Mol. Sci., 2012, 13, 8915–8932 CrossRef CAS PubMed.
- L. Fernández and R. E. W. Hancock, Clin. Microbiol. Rev., 2012, 25, 661–681 CrossRef PubMed.
- E. C. Smith, G. W. Kaatz, S. M. Seo, N. Wareham, E. M. Williamson and S. Gibbons, Antimicrob. Agents Chemother., 2007, 51, 4480–4483 CrossRef CAS PubMed.
- S. Gibbons, M. Oluwatuyi and G. W. Kaatz, J. Antimicrob. Chemother., 2003, 51, 13–17 CrossRef CAS PubMed.
- M. Chatterjee, C. P. Anju, L. Biswas, V. Anil Kumar, C. Gopi Mohan and R. Biswas, Int. J. Med. Microbiol., 2016, 306, 48–58 CrossRef CAS PubMed.
- P. S. Stewart, Int. J. Med. Microbiol., 2002, 292, 107–113 CrossRef CAS PubMed.
- M. Monzón, C. Oteiza, J. Leiva and B. Amorena, J. Antimicrob. Chemother., 2001, 48, 793–801 CrossRef.
- P. Y. Chung, P. Navaratnam and L. Y. Chung, Ann. Clin. Microbiol. Antimicrob., 2011, 10, 1–6 CrossRef PubMed.
- A. Kurek, A. M. Grudniak, M. Szwed, A. Klicka, L. Samluk, K. I. Wolska, W. Janiszowska and M. Popowska, Antonie van Leeuwenhoek, 2009, 97, 61–68 CrossRef PubMed.
- S. Fontanay, M. Grare, J. Mayer, C. Finance and R. E. Duval, J. Ethnopharmacol., 2008, 120, 272–276 CrossRef CAS PubMed.
- S.-G. Kim, M. J. Kim, D. Jin, S.-N. Park, E. Cho, M. O. Freire, S.-J. Jang, Y.-J. Park and J.-K. Kook, Korean J. Microbiol., 2012, 48, 212–215 CrossRef.
- A. Kurek, P. Nadkowska, S. Pliszka and K. I. Wolska, Phytomedicine, 2012, 19, 515–519 CrossRef CAS PubMed.
- D. Abramson, L. J. Goad and T. W. Goodwin, Phytochemistry, 1973, 12, 2211–2216 CrossRef CAS.
- I. E. Orhan, S. A. Erdem, F. S. Senol, M. Kartal and B. Sener, Ind. Crops Prod., 2012, 40, 116–121 CrossRef CAS.
- B. Shin and W. Park, PLoS One, 2015, 10, e0137751 Search PubMed.
- K. Wolska, A. Grudniak, B. Fiecek, A. Kraczkiewicz-Dowjat and A. Kurek, Open Life Sci., 2010, 5, 543–553 CAS.
- S. Perumal and R. Mahmud, BMC Complementary Altern. Med., 2013, 13, 1–8 CrossRef PubMed.
- N. Qin, X. Tan, Y. Jiao, L. Liu, W. Zhao, S. Yang and A. Jia, Sci. Rep., 2014, 4, 5467 CAS.
- D. Ren, R. Zuo, A. F. González Barrios, L. A. Bedzyk, G. R. Eldridge, M. E. Pasmore and T. K. Wood, Appl. Environ. Microbiol., 2005, 71, 4022–4034 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21137b |
| This journal is © The Royal Society of Chemistry 2016 |