Catalytic removal of organic colorants from water using some transition metal oxide nanoparticles synthesized under sunlight†
Uma Shanker*,
Vidhisha Jassal and
Manviri Rani
Department of Chemistry, Dr B R Ambedkar National Institute of Technology, Jalandhar, Punjab, India-144011. E-mail: shankeru@nitj.ac.in; umaorganic29@gmail.com; Fax: +91-181-269-0932; Tel: +91-7837-588-168 Tel: +91-181-269-301-2258
First published on 20th September 2016
Abstract
Transition metal oxides (TMO) constitute a most amazing class of materials with a wide range of properties and applications; therefore, their synthesis using a green approach is a necessity. As such, sunlight irradiation was employed to synthesize various TMO nanostructures (ZnO, CuO, Co3O4, NiO and Cr2O3) using water as a solvent. Nanoparticles obtained with distinct morphologies, such as nanotubes (ZnO; <35 nm), nanorods (CuO; 7–50 nm), nanorods, triangles and hexagons (Co3O4; 45–90 nm), needle-shaped (NiO; 2–25 nm) and nanobeads (Cr2O3; ∼17 nm) were confirmed by TEM analysis. The significance of the synthesis is in its quick approach with no thermal heat involvement, reusable catalyst, cost effectiveness and ability to fabricate almost uniformly distributed nanoparticles with small sizes. The potential of the synthesized nanoparticles was examined in the treatment of simulated water containing hazardous dyes: Alizarin Red S (ARS) + Methylene Blue (MB). Interestingly, in a short period of 180 min, 88.24% of the dye mixture was, for the most part, completely degraded using Cr2O3 nano-needles, followed by 87.96% (ZnO) > 86.86% (CuO) > 85.89% (NiO) > 80.35% (Co3O4), depending on the sizes of the respective TMO nanoparticles. This is also supported by the finding of small and non-toxic by-products such as but-2-enal, sulfur trioxide and benzoquinone. With high potential observed in the removal of dyes, TMO nanoparticles have a bright future with respect to their use as important adsorbents in waste water treatment. The advantage of the present work lies in the green synthesis of nanoparticles and their application in helping to make our environment green.
1. Introduction
Transition metal oxides (TMOs) are an important class of materials, due to their attractive magnetic, electronic and optical properties.1,2 This makes them effective for use in a variety of applications, such as catalysis,3 sensors,4 lithium ion batteries4,5 and many more.6,7 Concerning these applications, TMOs are advantageous in comparison to the other conventionally used materials. Therefore, their synthesis using advanced methods is of key interest to researchers around the globe.In general, various methods based on dry and wet procedures have been developed for the synthesis of TMO nanoparticles.8,9 However, such methods have disadvantages in terms of temperature and the requirement for toxic chemical reagents that may harm the environment. To overcome these problems, the best possible way is to move towards green chemistry, i.e., avoiding the use of hazardous reagents and replacing them with the natural ones in all the technological processes.10 Along with the reduction in environmental pollution,11 green routes yield contaminant free nanoparticles with small size and distinct morphologies.12,13 Following the green chemistry principles, the authors decided to employ a sunlight assisted approach to the synthesis of various TMO nanoparticles, namely, zinc oxide (ZnO), chromium oxide (Cr2O3), cobalt oxide (Co3O4), copper oxide (CuO) and nickel oxide (NiO) nanoparticles.
Recently, the applications of TMO nanoparticles in the treatment of wastewater containing organic colorants have gained immense interest, owing to their high surface area and semiconducting properties.14 Irregular and unsystematic use of dyestuffs is glaring evidence of environmental pollution. Textile and paper industries discharge an enormous volume of pollutants, non-degradable and carcinogenic, colored dye effluents into the water bodies without any proper treatment, thus leading to the contamination of water resources.15 Therefore, it is a matter of utmost importance to eliminate the dyestuffs from water bodies. Recently, the authors reported the degradation of malachite green and eriochrome black T using potassium zinc hexacyanoferrate nanoparticles.16 This motivated us to explore the effective use of other nanoparticles, such as TMO, in the degradation/removal of hazardous organic dyes.
The dye Alizarin Red S (ARS) has been used considerably in the textile industry since ancient times.17 ARS (1,2-dihydroxy-9,10-anthra-quinonesulfonic acid sodium salt) is a water-soluble, widely used anthraquinone dye with carcinogenic effects, and its release into the environment can cause serious problems to human beings and animals. ARS is resistant to degradation because of its complex aromatic structure, high thermal and optical stability. Therefore, an effective catalyst is required for the degradation of ARS.18 Methylene Blue (MB) is widely used in coatings for paper stock, coloring paper, and dyeing cottons and wools, etc. Acute exposure to MB will result in increased heart rate, vomiting, shock, Heinz body formation, cyanosis, jaundice, quadriplegia and tissue necrosis in humans.19,20 Due to their wide use, toxicity, high persistence and bioaccumulation, the authors selected these two dyes to observe the potential of synthesized TMO nanoparticles to remove them from simulated contaminated water.
The green synthesis of various TMO nanoparticles was carried out using the sunlight irradiation technique. In this method, the metal salt solution was exposed to sun light for one hour, followed by the addition of aqueous NaOH solution. The immediate precipitation of metal oxide nanoparticles occurred. Further characterization revealed the well-defined morphologies and uniform distribution of nanoparticles. The green synthesized TMO nanoparticles were employed for the treatment of simulated water containing the ARS + MB dye mixture, which constitutes the hazardous discharge from industries into the water bodies. Various process parameters, such as the concentration of the mixed dye solution, dose amount of catalysts used and the pH of mixed dye solution were optimized. To the best of our knowledge, no such method has been reported before, for the sunlight assisted, green synthesis of TMO nanoparticles and their use in the removal of these dyes from simulated water.
2. Experimental
Zinc nitrate (Zn(NO3)2), copper nitrate (Cu(NO3)2), cobalt nitrate (Co(NO3)2), nickel nitrate (Ni(NO3)2), chromium nitrate (Cr(NO3)3) and sodium hydroxide (NaOH) used in this study were of analytical grade and procured from Merck. All solutions were prepared using double distilled water.2.1 Design of experiment
Reactions were investigated both with and without photo-illumination. No precipitation was observed without photo-illumination, whereas, immediate precipitation of the TMO nanoparticles was observed within 1 hour of photo-illumination. The reason might be the presence of a greater number of photons of certain wavelength in direct sunlight that initiate the reaction, thus promoting the formation of TMO nanoparticles.21–23
Initially the metal nitrate reacts with the sodium hydroxide to form [M(OH)x]+, as shown below:
| M(NO3)x + NaOH → [M(OH)x]+ |
When the aqueous mixture is kept under sunlight irradiation, the following process occurs:
| H2O → e− + H3O+ + H2 + ˙H + ˙OH + H2O2 |
The solvated electrons and free radicals are highly active species that help in generating TMO nanoparticles.
| [M(OH)x]+ + e− + ˙OH → MxOy + H2O |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The absorbance spectrum of the supernatant was subsequently measured, and this continued for up to 180 minutes.
000 rpm for 10 min. The absorbance spectrum of the supernatant was subsequently measured, and this continued for up to 180 minutes.Different parameters (Fig. 5), such as catalyst amount, initial concentration and the initial pH of the mixed dye solution were optimized in order to get the maximum dye degradation (%) by the synthesized TMO nanoparticles. These collected samples were also analyzed for the identification of possible degradation products.
2.2 Instrumentation
Powder X-ray diffraction (PXRD) was carried out on a PANalytical X'PERT PRO instrument using CuKα (λ = 1.5406 Å) radiation. Field emission scanning electron microscopy (FE-SEM) was carried out in order to determine the surface morphology and average particle size of the synthesized TMO nanoparticles (Quanta 200 FEG). Exact particle size and shape of the nanoparticles were determined using transmission electron microscopy (TEM), using a Hitachi (H-7500) instrument operating at 120 kV. A Malvern Zetasizer (Nano ZS) instrument was used to measure the zeta potential of the nanoparticles.A spectrophotometer (Agilent Pro) was used for the quantitative measurement of dyes. A gas chromatograph, GC 1300, coupled with a mass spectrometer, TSQ8000, was used for the identification of oxidative degradation products. The column used was TG 5MS having dimensions 30 m × 0.2 mm × 0.2 mm. The conditions for GC were as follows: injector temperature, 280 °C and transfer line temperature, 250 °C. The capillary column temperature was programmed to 80 °C for 2 min, from 80 to 260 °C at 10 °C min−1 and held at 250 °C for 15 min. Helium was used as a carrier gas, with a flow rate of 2 mL min−1. The mass spectrometer conditions were ion source at 250 °C and ionization energy of 40 eV.
3. Results and discussion
Employing a green pathway based on sunlight and water, various TMO nanoparticles were successfully synthesized. Their detailed structural morphologies and potential for the degradation of hazardous dyes mixture are discussed. The PXRD, FE-SEM/EDS and TEM images are shown in Fig. 1–3, respectively.3.1 Powder X-ray diffraction
PXRD data were found to be concordant with the JCPDS data (ZnO Card no. 79-2205, CuO Card no. 80-1917, Co3O4 Card no. 76-1802, NiO Card no. 22-1189, Cr2O3 Card no. 76-0147). The peaks of the XRD spectra confirm the formation of the respective products (Fig. 1). The symmetry of the as synthesized TMO nanoparticles was obtained as follows: hexagonal (ZnO), monoclinic (CuO), cubic (Co3O4), rhombohedral (NiO) and rhombohedral (Cr2O3). The absence of secondary peaks indicates the high purity level of the samples. In the case of ZnO, CuO, Co3O4, NiO and Cr2O3, the maximum relative intensities (%) were observed at 17.47°, 25.26°, 25.04°, 25.02° and 33.67° on the 2θ scale, respectively (Table 1S†). A comparison was also carried out with traditionally used methods to confirm the effectiveness of the current approach. Nugroho and Kim (2014) synthesized Co3O4 nanoparticles with high crystal size and low crystallinity at low concentrations of KOH, using supercritical water.24 A thermal treatment method was also employed to synthesize crystalline Co3O4 nanoparticles with no impurity peaks.25 Various other methods have also been reported for the synthesis of cobalt oxide nanoparticles, such as mechanochemical, micro-emulsion, etc.26,27 Employing a sol–gel technique, NiO nanoparticles having an average size of 5 nm and rock-salt structure were synthesized.28 Heat-treated, cubic nickel oxide nanoparticles with a high level of purity have also been synthesized.29,30 Gibot and Vidal synthesized crystalline Cr2O3 nanoparticles (with no impurities of silica) using Cr(III) nitrate and nanometric silica spheres as precursor and template agent, respectively.31 Magnetic chromium oxide nanoparticles were also synthesized via this simple approach.32 Through the electrochemical method, ZnO nanoparticles with high crystallinity were synthesized.33 Various other techniques, like micro-emulsion, thermal treatment, etc., also resulted in monodisperse ZnO nanoparticles.34–36 Good crystallinity of CuO nanoparticles was obtained using Cu(NO3)2 as the precursor, compared to the CuCl2 precursor, with an increase in crystallinity after annealing.37 A thermal decomposition route was employed for synthesizing copper oxide nanoparticles with a high purity level.38 Using carbon nanotubes as a template, Wu et al. (2002) synthesized CuO nanoparticles with average size of ∼37.2 nm, as confirmed by XRD analysis.39 Though our results are similar to the conventional methods, such as electrochemical or sol–gel, in providing the nanosized, crystalline TMOs, the green approach is best because it is easy to perform and is a good alternative to the earlier time consuming and costly methods.3.2 FE-SEM
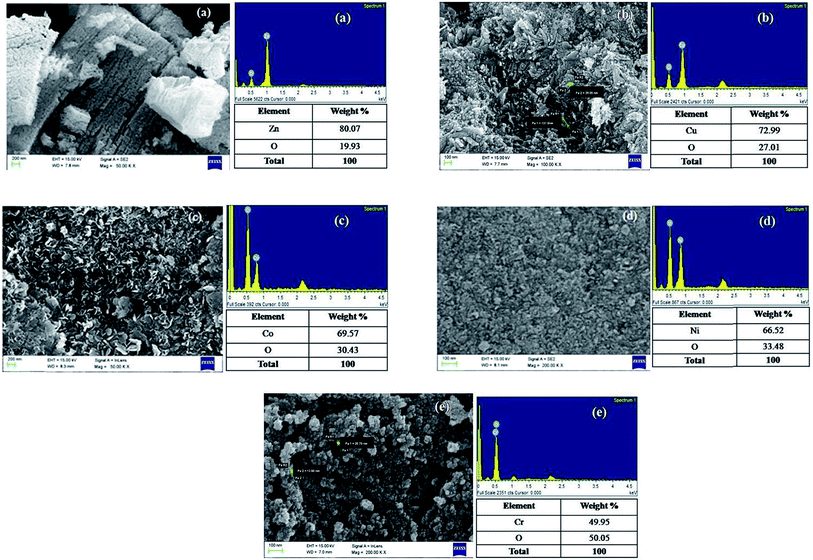
It is interesting to note here that even though all the TMOs were synthesized via the same method, they exhibited different morphologies and were distributed almost uniformly throughout the network (Fig. 2). Long, uniform channels of ZnO nanoparticles (<50 nm) were formed in the shape of tubes. Long nanoparticle rods of CuO having average size less than 100 nm were observed. Almost hexagonal shaped Co3O4 nanoparticles (50–100 nm) were formed. The average particle size in the case of NiO and Cr2O3 nanoparticles was extremely small (<10 nm).The EDS (Energy Dispersive X-ray Spectroscopy) analyses of all the samples indicate the presence of oxygen, along with the concerned metal ion (Fig. 2). In ZnO, Zn and O showed 80.07 and 19.93 weight (%), and 49.58 and 50.42 atomic (%), respectively. In the case of CuO, the weight (%) for Cu and O were 72.99 and 27.01, respectively, whereas atomic (%) values were found to be 40.49 and 59.51, respectively. In Co3O4, Co and O exhibited 69.57 and 30.43 weight (%); the atomic (%) were found to be 38.29 and 61.71, respectively. Also, in the case of NiO, the weight (%) of Ni and O correspond to 66.52 and 33.48, and the atomic (%) were 35.13 and 64.87, respectively. In Cr2O3, Cr and O exhibited 49.95 and 50.05 weight (%) and 23.49 and 76.51 atomic (%), respectively.
3.3 TEM
Small nanotubes of ZnO having size less than 35 nm were formed. The attractive morphology of CuO, i.e., long nanorods (diameter 7–50 nm), was observed. It is important to note that in the case of Co3O4, three types of nanostructures (namely, nanorods, triangular and hexagonal shaped), were observed. The length of the nanorods varied between 50–100 nm, whereas the diameter was less than 50 nm. The particle sizes of the triangular and hexagonal shaped Co3O4 nanoparticles were also less than 50 nm. Needle-shaped NiO nanoparticles having extremely small sizes (length and width less than 25 and 2 nm, respectively) were formed. Moreover, nanobeads of Cr2O3 were formed with size of ∼17 nm. The most interesting feature of this study was the formation of various TMO nanoparticles with unique morphologies and sizes less than 100 nm (Fig. 3).3.4 Application of TMO nanostructures in the removal of hazardous dyes
The photocatalytic activity of the above synthesized TMO nanoparticles was evaluated for the removal of a mixture of two dyes, namely, ARS and MB. Concentration was found to decrease continuously with the increase in the time interval. Under optimized conditions, the photocatalytic removal capacity of various nanostructures was observed as follows: Cr2O3 (88.24%) > ZnO (87.96%) > CuO (86.86%) > NiO (85.89%) > Co3O4 (80.35%), depending on the size of the nanoparticles formed.Previous studies reported by researchers show that the maximum dye (Congo Red) degradation of 81.33% was achieved using ZnO nanoparticles synthesized via the sol–gel method.40 Also, the degradation efficiency of ∼72% was achieved using ZnO as a photocatalyst.41 Methyl violet was degraded by 87% using ZnO nanoparticles in the precipitation technique.42 The degradation of ARS under UV light irradiation was carried out using ZnO nanoparticles (∼77% degradation in 90 minutes).43 Moreover, CuO nanostructures synthesized via the hydrothermal route were able to degrade methylene blue only in the presence of H2O2 as an oxidizing agent.44 This proves the effectiveness of the above synthesized nanoparticles, which can degrade dyes in the absence of any external oxidizing agent. Vaseem et al. (2008) utilized CuO nanoparticles for degrading MB dye. Results revealed the lower catalytic efficiency of the catalyst as a negligible amount of dye got degraded.45 Thus, it is clear from the above discussion that TMO nanoparticles synthesized via the sunlight mediated green route, have better catalytic efficiencies for the removal of organic dyes than the conventional TMO nanoparticles. Also, these nanomaterials are cost-effective, and can therefore be employed on a larger scale for degrading hazardous organic dyes.
3.5 Reaction mechanism
TMOs (ZnO, CuO, Co3O4, NiO and Cr2O3) being semiconducting in nature, are able to generate electron–hole pairs (in the conduction and valence band, respectively) upon photoillumination. The interaction of these electron–hole pairs with water produces active OH˙ species, which break the large, harmful organic dyes (Fig. 6). First, adsorption of the dye takes place on the surface of the adsorbent (TMO nanoparticles), followed by the dye degradation process.54 Overall, the generation of electron–hole pairs with OH˙ is responsible for the photodegradation of different organic dyes, as well as other organic pollutants. Electrons in the conduction band on the surface of the catalyst can reduce molecular oxygen to superoxide ions, which ultimately form hydrogen peroxide. The holes generated in situ are responsible for the generation of hydroxyl radicals, which are the primary agents responsible for the cleavage of dye mineralization.47or
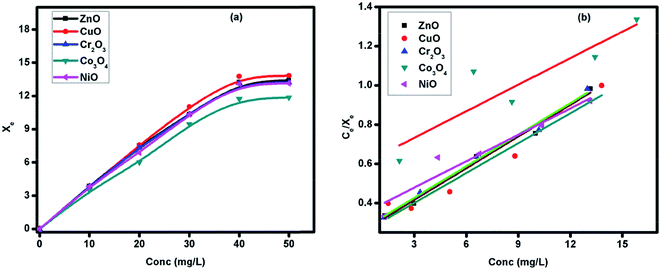
where, Ce is the equilibrium concentration of the dye solution; Xe is the amount of dye adsorbed per gram of adsorbent; Xm is the amount of dye adsorbed at saturation; kL is the Langmuir adsorption constant. Xm, kL and R2 values were determined to be: ZnO (18.587; 4.744; 0.985), CuO (19.696; 4.896; 0.933), Cr2O3 (18.663; 4.969; 0.988), Co3O4 (22.183; 13.254; 0.777) and NiO (22.306; 7.686; 0.905).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond functional group. Attack by OH˙ on this functional group leads to the cleavage of bonds and 2a (3-(3-(dimethylamino)cyclohexa-2,4-dien-1-yl)sulfinyl-N,N-dimethylbenzene-1,4diamine) is generated, having m/z = 303. The oxidation state of S changes from −2 to 0 on the attack of OH˙. This conversion to C–S(
C bond functional group. Attack by OH˙ on this functional group leads to the cleavage of bonds and 2a (3-(3-(dimethylamino)cyclohexa-2,4-dien-1-yl)sulfinyl-N,N-dimethylbenzene-1,4diamine) is generated, having m/z = 303. The oxidation state of S changes from −2 to 0 on the attack of OH˙. This conversion to C–S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–C ultimately leads to the opening of the central aromatic ring. The subsequent attack of OH˙ finally leads to the formation of an intermediate (2b; phenol), with the release of NH4+ and SO42− ions. The intermediate (2b) then gets converted into benzoquinone (2c). This is further supposed to get mineralized into stable CO2.57 Degradation of MB also results in the formation of 2H-1,4-thiazene (3) having m/z = 98, which ultimately gets mineralized into CO2, NO3−, NH4+ and SO42− ions. Attack of active OH˙ species can also take place at the C-5 position, leading to the formation of the intermediate 4 (3,4,5-trihydroxy-9,10-dioxo-dihydroanthracene-2-sulfonic acid), which gets converted into 6-formyl-3,4-dihydroxy-5,8-dioxo-5,8-dihydronaphthalene-2-sulphonic acid (4a; m/z = 295). This ultimately gets cleaved into but-2-enal (4b; m/z = 71). In another proposed pathway for the degradation of MB, an intermediate 5 (3,7-bis(dimethylamino)-9-formyl-4aH-phenothiazine-1-carboxylic acid) having m/z = 355 is generated, which after a series of oxidative and bond cleavages, results in the formation of 5a ((Z)-2-aminoethenethiol; m/z = 73). This species is also supposed to get mineralized into NO3−, NH4+ and SO42− ions. In addition to this, various other by-products are also formed, such as sulfur trioxide (6; m/z = 83), 2,3-dimethyl cyclohexa-2,5-diene-1,4-dione (7; m/z = 140) and benzoquinone (2c; m/z = 108). Formation of CO2, NO3−, NH4+ and SO42− ions suggest complete mineralization of the dye mixture.
O)–C ultimately leads to the opening of the central aromatic ring. The subsequent attack of OH˙ finally leads to the formation of an intermediate (2b; phenol), with the release of NH4+ and SO42− ions. The intermediate (2b) then gets converted into benzoquinone (2c). This is further supposed to get mineralized into stable CO2.57 Degradation of MB also results in the formation of 2H-1,4-thiazene (3) having m/z = 98, which ultimately gets mineralized into CO2, NO3−, NH4+ and SO42− ions. Attack of active OH˙ species can also take place at the C-5 position, leading to the formation of the intermediate 4 (3,4,5-trihydroxy-9,10-dioxo-dihydroanthracene-2-sulfonic acid), which gets converted into 6-formyl-3,4-dihydroxy-5,8-dioxo-5,8-dihydronaphthalene-2-sulphonic acid (4a; m/z = 295). This ultimately gets cleaved into but-2-enal (4b; m/z = 71). In another proposed pathway for the degradation of MB, an intermediate 5 (3,7-bis(dimethylamino)-9-formyl-4aH-phenothiazine-1-carboxylic acid) having m/z = 355 is generated, which after a series of oxidative and bond cleavages, results in the formation of 5a ((Z)-2-aminoethenethiol; m/z = 73). This species is also supposed to get mineralized into NO3−, NH4+ and SO42− ions. In addition to this, various other by-products are also formed, such as sulfur trioxide (6; m/z = 83), 2,3-dimethyl cyclohexa-2,5-diene-1,4-dione (7; m/z = 140) and benzoquinone (2c; m/z = 108). Formation of CO2, NO3−, NH4+ and SO42− ions suggest complete mineralization of the dye mixture.
4. Reusability of catalyst
To test the reusability of the catalyst in the simulated water treatment, the decolorization reaction was run with a 180 min cycle. At the end of each cycle, TMO nanoparticles were separated, heated to 60 °C for 30 min, followed by washing with acetone, as well as distilled water, and further used for the next cycle. The results showed that TMO nanoparticles could be reused at least ten times (Fig. 1S†) without a significant loss in their adsorbing properties.5. Environmental concerns of hazardous dyes
Industries such as textiles, cosmetics, paper, printing, etc., involve the use of dyes in aqueous solutions and discharge them untreated into the water reservoirs. The amount of dyes discarded each year is constantly increasing, which is the major reason for environmental pollution. Moreover, the use of reactive dyes worldwide has increased from 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes in 1988 to 178
000 tonnes in 1988 to 178![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes in 2004.58 Clinical and immunological investigation revealed that about 15% of 400 workers exposed to reactive dyes experienced nasal and respiratory problems.59 Exposure to such dyestuffs causes serious health hazards, since they have a tendency to bind to the body's proteins.60 In early 1981, some sulphonyl ethyl sulphate derivatives were observed to be carcinogenic60 and various textile dyes and their raw materials, including benzidine and o-toluidine based compounds are included in the international register of cancer-causing chemicals.61
000 tonnes in 2004.58 Clinical and immunological investigation revealed that about 15% of 400 workers exposed to reactive dyes experienced nasal and respiratory problems.59 Exposure to such dyestuffs causes serious health hazards, since they have a tendency to bind to the body's proteins.60 In early 1981, some sulphonyl ethyl sulphate derivatives were observed to be carcinogenic60 and various textile dyes and their raw materials, including benzidine and o-toluidine based compounds are included in the international register of cancer-causing chemicals.61
In order to regulate this negative impact of dyes on living species, several methods, such as physical separation, chemical processes and biological degradation are being carried out. Due to the growing awareness worldwide, researchers are showing keen interest in the development of effective removal techniques. Some important techniques are widely used, including adsorption, oxidation, coagulation–flocculation, biological treatment, electrochemical treatment and membrane filtration. This needs further advancement so that it can be employed on a large scale.
6. Conclusions
A quick green approach using sunlight and water for the synthesis of ZnO, CuO, Co3O4, NiO and Cr2O3 nanostructures was successfully established. Almost uniformly distributed TMO nanoparticles were obtained with distinct morphologies, such as nanotubes (ZnO), nanorods (CuO), nanorods, triangles and hexagons (Co3O4), needle-shaped (NiO) and nanobeads (Cr2O3). These TMOs were found to be potential catalysts in the treatment of simulated water containing hazardous dyes: ARS + MB. The degradation efficiency of Cr2O3 was highest (10 mg L−1 of dye; 15 mg catalyst loading and neutral pH) followed by ZnO > CuO > NiO > Co3O4, depending on the size of the respective TMO. The finding of small and non-toxic by-products like but-2-enal, sulfur trioxide and benzoquinone indicates the positive aspects of using synthesized nanoparticles in the removal of toxic dyes. Some oxidative by-products, such as 2H-1,4-thiazene-2,3,6-triol, 2H-1,4-thiazene, 2,3-dimethylcyclohexa-2,5-diene-1,4-dione, maleic acid and malealdehyde were identified. The further spread of TMO nanoparticle utilization in waste water treatment for the removal of several other harmful pollutants, such as polycyclic aromatic hydrocarbons (PAHs), aromatic amines, various types of phenols and other carcinogenic materials, needs to be explored more effectively. In addition, the reusability of the catalyst can decrease the cost of the process.Acknowledgements
Authors are thankful to DST-FIST New Delhi for providing the equipment (UV-VIS spectrophotometer) used in characterization of the samples. Authors are also thankful to NIPER Mohali for Zeta potential analysis and SAIF Panjab University, Chandigarh for TEM analysis. One of the authors Ms Vidhisha Jassal is thankful to Ministry of Human Resource Development (MHRD), New Delhi for providing fellowship.References
- M. A. Laguna-Marco, D. Haskel, N. Souza-Neto, J. C. Lang, V. V. Krishnamurthy, S. Chikara, G. Cao and M. van Veenendaal, Phys. Rev. Lett., 2010, 105, 216407 CrossRef CAS PubMed.
- B. J. Kim, H. Jin, S. J. Moon, J. Y. Kim, B. G. Park, C. S. Leem, J. Yu, T. W. Noh, C. Kim, S. J. Oh, J. H. Park, V. Durairaj, G. Cao and E. Rotenberg, Phys. Rev. Lett., 2008, 101, 076402 CrossRef CAS PubMed.
- F. Jiao and H. Frei, Energy Environ. Sci., 2010, 3, 1018 CAS.
- W. Y. Li, L. N. Xu and J. Chen, Adv. Funct. Mater., 2005, 15, 851 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496 CrossRef CAS PubMed.
- X. H. Lu, J. Lei, D. Zhou, S. Y. Fang, Y. L. Dong and Q. H. Xia, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2010, 49, 1586 Search PubMed.
- M. Te and H. C. Foley, Appl. Catal., A, 1994, 119, 97 CrossRef CAS.
- X. Y. Deng and Z. Chen, Mater. Lett., 2004, 58, 276 CrossRef CAS.
- H. Mohebbi, T. Ebadzadeh and F. A. Hesari, J. Power Sources, 2008, 178, 64 CrossRef CAS.
- J. A. Linthorst, Found. Chem., 2010, 12, 55 CrossRef CAS.
- P. T. Anastas and J. B. Zimmerman, Why we need a green nano award & how to make it happen, Woodrow Wilson International Center for Scholars, Washington DC, 2007, p. 1 Search PubMed.
- J. E. Hutchison, ACS Nano, 2008, 2, 395 CrossRef CAS PubMed.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940 CrossRef CAS PubMed.
- S. H. S. Chan, T. Y. Wu, J. C. Juan and C. Y. The, J. Chem. Technol. Biotechnol., 2011, 86, 1130 CrossRef CAS.
- T. Robinson, G. McMullan, R. Marchant and P. Nigam, Bioresour. Technol., 2004, 77, 247 CrossRef.
- V. Jassal, U. Shanker, B. S. Kaith and S. Shankar, RSC Adv., 2015, 5, 26141 RSC.
- S. Prillo, M. L. Ferreira and E. H. Rueda, J. Hazard. Mater., 2009, 168, 168 CrossRef PubMed.
- J. Kurepa, T. Paunesku, S. Vogt, H. Arora, B. M. Rabatic, J. Lu, M. B. Wanzer, G. E. Woloschak and J. A. Smalle, Nano Lett., 2010, 10, 2296 CrossRef CAS PubMed.
- K. V. Kumar, V. Ramamurthi and S. Sivanesan, J. Colloid Interface Sci., 2005, 284, 14 CrossRef CAS PubMed.
- V. Vadivelan and K. V. Kumar, J. Colloid Interface Sci., 2005, 286, 90 CrossRef CAS PubMed.
- S. M. Shaban, I. Aiad, M. M. Sukkary, E. A. Soliman and M. Y. Awady, Chin. Chem. Lett., 2015, 26, 1415 CrossRef CAS.
- G. A. Bhaduri, R. Little, R. B. Khomane, S. U. Lokhande, B. D. Kulkarni, B. G. Mendis and L. Siller, J. Photochem. Photobiol., A, 2013, 258, 1 CrossRef CAS.
- M. Guan, Z. Zhou, R. Duan, B. Du, X. Li, L. Liu and Q. Zhang, RSC Adv., 2015, 5, 26540 RSC.
- A. Nugroho and J. Kim, J. Ind. Eng. Chem., 2014, 20, 4443 CrossRef CAS.
- M. Salavati-Niasari, A. Khansari and F. Davar, Inorg. Chim. Acta, 2009, 362, 4937 CrossRef CAS.
- H. Yang, Y. Hu, X. Zhang and G. Qiu, Mater. Lett., 2004, 58, 387 CrossRef CAS.
- J. Ahmed, T. Ahmad, K. V. Ramanujachary, S. E. Lofland and A. K. Ganguli, J. Colloid Interface Sci., 2008, 321, 434 CrossRef CAS PubMed.
- M. Tadic, D. Nikolic, M. Panjan and G. R. Blake, J. Alloys Compd., 2015, 647, 1061 CrossRef CAS.
- M. Salavati-Niasari, F. Davar and Z. Fereshteh, J. Alloys Compd., 2010, 494, 410 CrossRef CAS.
- N. Dharmaraj, P. Prabu, S. Nagarajan, C. H. Kim, J. H. Park and H. Y. Kim, Mater. Sci. Eng., B, 2006, 128, 111 CrossRef CAS.
- P. Gibot and L. Vidal, J. Eur. Ceram. Soc., 2010, 30, 911 CrossRef CAS.
- N. R. Jana, Y. Chen and X. Peng, Chem. Mater., 2004, 16, 3931 CrossRef CAS.
- V. Anand and V. C. Srivastava, J. Alloys Compd., 2015, 636, 288 CrossRef CAS.
- S. Hingorani, V. Pillai, P. Kumar, M. S. Multani and D. O. Shah, Mater. Res. Bull., 1993, 28, 1303 CrossRef CAS.
- L. Wang and M. Muhammed, J. Mater. Chem., 1999, 9, 2871 RSC.
- A. Becheri, M. Dürr, P. L. Nostro and P. Baglioni, J. Nanopart. Res., 2008, 10, 679 CrossRef CAS.
- K. Phiwdanga, S. Suphankija, W. Mekprasarta and W. Pecharapa, Energy Procedia, 2013, 34, 740 CrossRef.
- M. Salavati-Niasari and F. Davar, Mater. Lett., 2009, 63, 441 CrossRef CAS.
- H. Q. Wu, X. W. Wei, M. W. Shao, J. S. Gu and M. Z. Qu, Chem. Phys. Lett., 2002, 364, 152 CrossRef CAS.
- C. B. Ong, A. W. Mohammad, R. Rohani, M. M. Ba-Abbad and N. H. H. Hairom, Process Saf. Environ. Prot., 2016 DOI:10.1016/j.psep.2016.04.006.
- N. H. H. Hairom, A. W. Mohammad and A. A. H. Kadhum, Sep. Purif. Technol., 2014, 137, 74 CrossRef CAS.
- K. Jeyasubramanian, G. S. Hikku and R. K. Sharma, Journal of Water Process Engineering, 2015, 8, 35 CrossRef.
- S. K. Kansal, R. Lamba, S. K. Mehta and A. Umar, Mater. Lett., 2013, 106, 385–389 CrossRef CAS.
- M. U. A. Prathap, B. Kaur and R. Srivastava, J. Colloid Interface Sci., 2012, 370, 144 CrossRef PubMed.
- M. Vaseem, A. Umar, Y. B. Hahn, D. H. Kim, K. S. Lee, J. S. Jang and J. S. Lee, Catal. Commun., 2008, 10, 11 CrossRef CAS.
- A. D. Bokare, R. C. Chikate, C. V. Rode and K. M. Paknikar, Appl. Catal., B, 2008, 79, 270 CrossRef CAS.
- N. Daneshvar, D. Salari and A. R. Khataee, J. Photochem. Photobiol., A, 2004, 162, 317 CrossRef CAS.
- X. Zhang, Y. Wang and G. Li, J. Mol. Catal. A: Chem., 2005, 237, 199 CrossRef CAS.
- M. A. Tariq, M. Faisal and M. Muneer, J. Hazard. Mater., 2005, 127, 172 CrossRef PubMed.
- L. Mu and S. S. Feng, J. Controlled Release, 2001, 76, 239 CrossRef CAS PubMed.
- R. Sankar, A. Karthik, A. Prabu, S. Karthik, K. S. Shivashangari and V. Ravikumar, Colloids Surf., B, 2013, 108, 80 CrossRef CAS PubMed.
- L. Lin, P. Qiu, X. Cao and L. Jin, Electrochim. Acta, 2008, 53, 5368 CrossRef CAS.
- M. B. Kasiri and H. Aleboyeh, Appl. Catal., B, 2008, 84, 9 CrossRef CAS.
- V. Jassal, U. Shanker and B. S. Kaith, Scientifica, 2016, 2016, 1 CrossRef PubMed.
- S. Viladkar, T. Alam and Kamaluddin, J. Inorg. Biochem., 1994, 53, 69 CrossRef CAS.
- G. Liu, X. Li, J. Zhao, S. Horikoshi and H. Hidaka, J. Mol. Catal. A: Chem., 2000, 153, 221 CrossRef CAS.
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J. M. Herrmann, Appl. Catal., B, 2001, 31, 145 CrossRef CAS.
- D. Phillips, J. Soc. Dyers Colour., 1996, 112, 183 CrossRef CAS.
- A. Docker, J. M. Wattie, M. D. Topping, C. M. Luczynska, A. J. N. Taylor, C. A. C. Pickering, P. Thomas and D. Gompertz, Br. J. Ind. Med., 1987, 44, 534 CAS.
- T. Keneklis, Fiber reactive dye toxicological profiles, U.S. Consumer Product Safety Commission, Washington D.C., Contact No. CPSC-C-81-1110, 1981, p. 271 Search PubMed.
- IARC, Benzidine-based dyes, IARC Monographs on evaluation of carcinogenic risk to humans, IARC, Lyon, 1987, p. 29 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17555d |
| This journal is © The Royal Society of Chemistry 2016 |