Anomalous red emission with competition and coexistence of defect and band edge emission in photo-electrochemically active (Zn0.97Ga0.03)(O0.95N0.05) solid solution†
Abstract
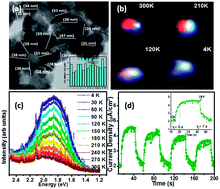
Photo-electrochemically active nanostructured (Zn0.97Ga0.03)(O0.95N0.05) solid solution has been synthesized by a solution combustion technique for realizing photocatalytic activity under visible light. A competing free excitonic and defect bound emission is observed from the sample which was investigated by temperature dependent photoluminescence from 4 K to 300 K. The defect bound emission dominates at room temperature providing an anomalous enhanced red emission, not reported before. A broad visible emission confirms the introduction of new defect levels as an impact of solid solution formation as established later by valence band (VB) XPS spectra. VB XPS shows the top of the valence band has shifted without affecting the conduction band, thereby reducing effective band gap of the solid solution from 3.35 eV to 2.8 eV. Tuning of the bandgap is essential to facilitate its activity under visible light irradiation as demonstrated in our present work. The generation of charge carriers, their effective separation and reduced trap states has been demonstrated by photoelectrochemical measurements in order to confirm the potential of the sample for efficient photocatalytic activity. The present sample exhibits a low mean life time with reduced trap states as compared to some previously reported results.

 Please wait while we load your content...
Please wait while we load your content...