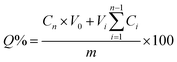Hydrogels generated by low-molecular-weight PEGylated luteolin and α-cyclodextrin through self-assembly for 5-fluorouracil delivery†
Weixia Qingab,
Yong Wanga,
Huan Lia,
Jinhua Zhua and
Xiuhua Liu*ac
aInstitute of Environmental and Analytical Sciences, College of Chemistry and Chemical Engineering, Henan University, Kaifeng, 475004, P. R. China. E-mail: ll514527@163.com
bMedical College, Henan University, Kaifeng, 475004, P. R. China
cKey Lab. of Natural Medicine and Immune-engineering of Henan Province, Henan University, Kaifeng, 475004, P. R. China
First published on 26th September 2016
Abstract
Hydrophobic luteolin (LUT) was conjugated to the oligomeric chain of methoxypoly(ethylene glycol) (mPEG) to form novel amphiphilic mPEG1900–LUT conjugates. Then mPEG1900–LUT, by using its adjacent 3′ and 4′ hydroxyl groups, were assembled with magnetic Fe3O4 particles in an aqueous solution to form mPEG1900–LUT–Fe3O4 conjugates, which formed hybrid Fe3O4 particles. The spectral properties and micellization of the conjugates were studied. Both mPEG1900–LUT and mPEG1900–LUT–Fe3O4 conjugates were able to self-assemble into stable supramolecular hydrogels through α-cyclodextrin (α-CD) in water, and their gelation times and temperatures were determined. The hydrogels displayed typical porous structures, which are suitable for drug delivery. Therefore, 5-fluorouracil (5-FU) was loaded into the formed hydrogels to control its release in vitro. The drug release observations showed that introducing Fe3O4 particles into the hydrogel improved the sustained release effect.
1. Introduction
Hydrogels have been recently utilized for controlled drug release, because of their excellent hydrophilicity, biocompatibility and low inflammatory response.1–3 Hydrogels composed of polyrotaxane constitute an important class of remarkable materials that are formed by a linear polymer chain threading through a series of cyclodextrin (CD) cavities.4–6 Polyrotaxane hydrogels have been reported to be endowed with tunable temperature-responsive potentials. So it is very beneficial to develop effective polyrotaxane hydrogels as delivery systems.7,8Poly(ethylene glycol) (PEG) has also been found to be able to thread through α-CD molecules of polyrotaxane.9–12 It has been quite generally stated in the literature that, a high molecular weight (MW > 10k) PEG is necessary for chemical and physical hydrogels. However, water-soluble polymer chains with high molecular weights (normally MW > 10k) are known to be unsuitable for filtration through the human kidney membrane because of their large hydrodynamic radii.13 Therefore, suitable procedures for preparing hydrogels based on low-MW PEGs and that can be used in pharmaceutical or medicinal applications are desirable for use as drug delivery and controlled release systems.
Luteolin (LUT, 3′,4′,5,7-tetrahydroxyflavone) is well known as a flavonoid, which mostly exists in various vegetables, fruits and medicinally important plants. LUT has been shown to display anticancer,14–17 anti-inflammatory,18 anti-allergic19 and anti-amnesic20 activities. Nevertheless, the application of LUT is hampered, due to its poor solubility.21
For the current work, we took into consideration the strong hydrophobicity of LUT to pursue the formation of novel amphiphilic conjugates using LUT as the hydrophobic part of the conjugate. LUT was linked to the terminal group of low-MW (1.9k) methoxy PEG (mPEG) to synthesize novel amphiphilic mPEG–LUT conjugates, which can self-assemble into nanoparticles with a core–shell structure in aqueous solutions. The mPEG–LUT conjugates, due to their having adjacent hydroxyl groups at the 3′ and 4′ positions, reacted with Fe3O4 particles to form hybrid nanoparticles. It is noteworthy that we succeeded in constructing hydrogels based on the complexes of α-CD and low-MW mPEG–LUT. At the same time, 5-FU was used as a model drug for investigating the performance of the hydrogels in drug loading and release.
2. Materials and methods
2.1. Chemicals and materials
LUT and mPEG (MW = 1.9k) were purchased from Sigma-Aldrich. Cesium carbonate, benzyl bromide, 4-toluene sulfonyl chloride and anhydrous dimethyl formamide were purchased from J&K Chemical Technology (Beijing China). All other chemicals were of analytical grade and supplied by Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).2.2. Preparation of Fe3O4 particles
FeCl3·6H2O, then sodium carboxymethylcellulose (CMC-Na) and finally sodium acetate anhydrous (NaAc) were dissolved in ethylene glycol. The mixture was transferred into a Teflon™-lined autoclave, kept at 200 °C for 10 h and then cooled to room temperature. The solid black products were collected and kept for later use on the basis of ref. 22.2.3. Synthesis of mPEG–LUT conjugates
2.4. Synthesis of mPEG–LUT–Fe3O4 conjugates
A mass of 15 mg of Fe3O4 particles was dispersed completely in 5 mL double-distilled water, to which a volume of 5 mL of the mPEG–LUT solution (4 mg mL−1) was then added under sonication for 40 minutes. Then the aqueous phase was separated, freeze-dried, and mPEG–LUT–Fe3O4 was obtained.2.5. Characterizations of the conjugates
The FTIR spectra were recorded on a Thermo Nicolet Avatar 360 spectrometer in transmittance mode using KBr pellets. UV-Vis spectroscopic studies were carried out using a TU-1900 spectrophotometer (Beijing Purkinje General Instrument Co. Ltd.). The saturation magnetization was obtained using an MPMS3 (Quantum Design, US). The zeta potential was recorded using a Malvern Nano-ZS90 instrument at 25 °C.2.6. Preparation of blank and 5-FU loaded hydrogels
One aqueous solution sample of α-CD (80.0 mg mL−1) was added to an aqueous solution of mPEG–LUT, and another to mPEG–LUT–Fe3O4, and for both resulting samples, the concentration was 10 mg mL−1. Each composition was mixed thoroughly by sonication for about 2 min followed by incubation at room temperature for 72 h before taking measurements. The gelation times of the supramolecular hydrogels were estimated using a vial-tilting method. The timer was started immediately after mixing the two components and the gelation time recorded was when no flow was observed for at least 1 min. Meanwhile, an α-CD (80.0 mg mL−1) solution and 5.0 mg of 5-FU were added to 1.0 mL of an aqueous solution of mPEG–LUT and mPEG–LUT–Fe3O4 (10 mg mL−1), respectively. Each resulting composition was mixed thoroughly by sonication for about 2 min followed by incubation at room temperature for 72 h before measurements were taken.2.7. In vitro drug release study
The 5-FU-loaded mPEG–LUT and mPEG–LUT–Fe3O4 supramolecular hydrogels were prepared in respective 1.5 mL reagent bottles. Each reagent bottle was placed upside-down in a dialysis bag with 15 mL of pH 7.4 phosphate-buffered (PBS) solution, which was incubated in 100 mL of PBS at 37 °C with gentle shaking. At predetermined time points, 3 mL of release medium was withdrawn and the same volume of fresh PBS solution was added to maintain a constant volume. The cumulative percentage release (Q%) was calculated using the equationwhere Cn (mg mL−1) is the concentration of drug in the sample, V0 (mL) is the volume of the release medium, Vi (mL) is the volume of the replaced medium, and m (mg) is the mass of the drug in the sample.
3. Results and discussion
3.1. Design and synthesis of mPEG–LUT conjugates
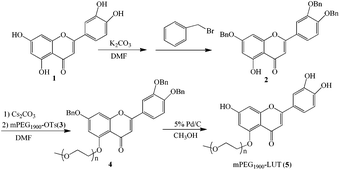
The syntheses of mPEG–LUT conjugates are shown in Scheme 1. LUT (1) was reacted with benzyl bromide to give 2, which is a crucial intermediate. During this synthetic process, the experimental results revealed the deprotonation of 1 with K2CO3 (4 equiv.) to be highly selective. Only the proton of the hydroxyl on the 5-position could not be removed. Coupling of 2 with 3 gave 4, which was deprotected with palladium(0) on carbon to give 5.3.2. Characterizations of conjugates
Both mPEG–LUT and mPEG–LUT–Fe3O4 conjugates had amphiphilic structures, which were able to self-assemble into stable micelles in aqueous solutions. As shown in Fig. S6,† the average sizes of mPEG–LUT and mPEG–LUT–Fe3O4 conjugate micelles were measured to be about 318.5 nm and 286.9 nm with polydispersity indexes of 0.310 and 0.348, respectively. Furthermore, the micellization of mPEG–LUT was studied by carrying out 1H-NMR spectroscopy in DMSO-d6 and D2O.23,24 DMSO was used as a non-selective solvent for both mPEG and LUT moieties, whereas water was used as a selective solvent that dissolved the mPEG well but was a poor solvent for LUT. In DMSO, both mPEG and LUT moieties gave rise to sharp peaks, indicative of their rapid molecular motions (Fig. 1A). However, in D2O, mPEG moieties gave a sharp peak, but LUT peaks were no longer sharp and were greatly reduced in intensity, indicating the formation of core/shell structures with relatively few LUT segments while the hydrophilic mPEG segments still moved freely in the water (Fig. 1B). The mPEG interacted with water molecules via hydrogen bonds, forming an exterior hydrophilic corona extending into the aqueous medium and stabilizing the micelle structure. The results confirmed that mPEG–LUT can self-assemble into a core/shell micelle structure in water.The FT-IR and UV-Vis spectra of both mPEG–LUT and mPEG–LUT–Fe3O4 conjugates are shown in Fig. 2. As seen Fig. 2A, an Fe–O bending vibration peak of Fe3O4 was observed at about 594 cm−1 in the spectrum of Fe3O4, but it shifted to 530 cm−1 in the spectrum of mPEG–LUT–Fe3O4 because the Fe3O4 reacted with the 3′ and 4′ adjacent hydroxyls. The Fe–O bending vibration peak of mPEG–LUT–Fe3O4 was very weak because of the low amount of Fe3O4 in the mPEG–LUT–Fe3O4; only 2.15% of the mPEG–LUT–Fe3O4 consisted of Fe3O4 according to Inductive Coupled Plasma Emission Spectrometer (ICP).
 | ||
| Fig. 2 FT-IR spectra (A) and UV-Vis spectra (B), and magnetization curves (C) of mPEG–LUT–Fe3O4 conjugates. | ||
As seen Fig. 2B, the ultraviolet absorption peak of mPEG–LUT at 354 nm sharply shifted to 379 nm after Fe3O4 reacted with the 3′ and 4′ adjacent hydroxyls. Meanwhile, analysis of the magnetization curves of mPEG–LUT–Fe3O4 conjugates, shown in Fig. 2C, indicated the saturation magnetization value to be about 2.2 emu g−1. This value is much smaller than the 80 emu g−1 value for Fe3O4 (see Fig. S1†), and this difference mainly derived from the different amount of Fe3O4 in the mPEG–LUT–Fe3O4 conjugates.
3.3. Formation of blank and 5-FU loaded hydrogels
A schematic representation of the supramolecular hydrogels made of mPEG–LUT (or mPEG–LUT–Fe3O4) conjugates and 5-FU with α-CD is shown in Scheme 2. Solutions of α-CD were added to mPEG–LUT and mPEG–LUT–Fe3O4 micelles, respectively, and the resulting compositions were mixed thoroughly by sonication. As expected, homogeneous hydrogels G1 and G2 gradually formed (Fig. 3). The hydrogels have been shown to be comprised of mPEG/α-CD complexes.25,26 Both α-CD and mPEG–LUT provided the supra-cross-links, which are particularly favorable to the gelation process. Both G1 and G2 exhibited a reversible gel–sol transition with a change in temperature. They started out mobile above a certain temperature, while they returned to an opaque gel phase after cooling to below this temperature. Meanwhile, G1 and G2 were found to be thermosensitive at about 40 °C, which is an acceptable temperature for site-specific drug delivery. In addition, introduction of Fe3O4 particles showed remarkable positive effects on gelation time, reducing it from 20 min to 5 min. Perhaps the Fe3O4 particles promoted the gelation process by contributing to the coordination interactions between the metal and polar groups. | ||
| Scheme 2 Schematic representation of the supramolecular hydrogels made of conjugates and 5-FU with α-CD. | ||
The hydrogels were kept for 72 h after formation, and freeze-dried under a vacuum at −54 °C for 24 h. Their morphologies were studied using a JSM-7001F (JEOL Co., Japan), as shown in the images of Fig. 4. G1 and G2 each clearly displayed a typical porous structure. Furthermore, after the introduction of Fe3O4 particles, G2 showed a flower-like structure, which may have been due to the increase in the network density.
3.4. In vitro drug release study
As shown in Fig. 5, the hydrogels G1 and G2 loaded with 5-FU showed controlled release properties. The 5-FU content was analyzed by monitoring the UV-Vis peak at a wavelength of 266.4 nm, and the assay method was validated. The calibration curve for 5-FU followed the equation A = 0.040 + 48.57C (R2 = 0.9991). In general, hydrogel release behavior is mainly influenced by diffusion and the breakup of supra-cross-links. In the current work, the introduction of Fe3O4 particles yielded a decrease in both the diffusion rate of the encapsulated 5-FU and in the breakup of interchain cross-links, and these decreases may have been due to the small pores and the high network density. The impact of the Fe3O4 particles was clearly considerable. Meanwhile, sustaining the release of 5-FU was found to be accompanied by releases of G1 and G2. Calculation of the cumulative percentage release of 5-FU allowed the cumulative percentage releases of mPEG–LUT and mPEG–LUT–Fe3O4 to be deduced.4. Conclusions
In conclusion, we have designed and synthesized novel mPEG–LUT and mPEG–LUT–Fe3O4 conjugates, which successfully self-assembled to form hydrogels system with α-CD. The conjugates displayed typical porous structures, and are hence suitable for drug delivery and tissue growth.27,28 We successfully developed classical drug-delivery polyrotaxane hydrogels system by introducing Fe3O4, which reduced the gelation time. Meanwhile, the formed hydrogels were able to load the anticancer drug 5-FU, and to achieve a targeted release of 5-FU. Such a flexible design provides a new, efficient and mild approach, and this novel system can be applied for the site-specific delivery of drugs using changes in temperature as a trigger.Acknowledgements
This work was supported by the Support Plan of Science and Technology Innovation team in Universities and Colleges in Henan Province of China (No. 14IRTSTHN030), Key Project of Science and Technology Research in Education Department of Henan Province in China (No. 14A150011) and Key Technology Research Program of Henan Province in China (No. 152102210257).Notes and references
- D. Limón, E. Amirthalingam, M. Rodrigues, L. Halbaut, B. Andrade, M. L. Garduño-Ramírez, D. B. Amabilino, L. Pérez-García and A. C. Calpena, Novel nanostructured supramolecular hydrogels for the topical delivery of anionic drugs, Eur. J. Pharm. Biopharm., 2015, 96, 421–436 CrossRef PubMed.
- X. F. Cheng, Y. Jin, T. B. Sun, R. Qi, H. P. Li and W. H. Fan, An injectable, dual pH and oxidation-responsive supramolecular hydrogel for controlled dual drug delivery, Colloids Surf., B, 2016, 141, 44–52 CrossRef CAS PubMed.
- Z. L. Zhang, Z. F. He, R. Liang, Y. Ma, W. J. Huang, R. Jiang, S. Shi, H. Chen and X. Y. Li, Fabrication of a micellar supramolecular hydrogel for ocular drug delivery, Biomacromolecules, 2016, 17, 798–807 CrossRef CAS PubMed.
- M. Ceccato, P. L. Nostro and P. Baglioni, α-Cyclodextrin/polyethylene glycol polyrotaxane:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a study of the threading process, Langmuir, 1997, 13, 2436–2439 CrossRef CAS.
a study of the threading process, Langmuir, 1997, 13, 2436–2439 CrossRef CAS. - J. Li and X. J. Loh, Cyclodextrin-based supramolecular architectures: syntheses, structures, and applications for drug and gene delivery, Adv. Drug Delivery Rev., 2008, 60, 1000–1017 CrossRef CAS PubMed.
- A. Harada, A. Hashidzume, H. Yamaguchi and Y. Takashima, Polymeric rotaxanes, Chem. Rev., 2009, 109, 5974–6023 CrossRef CAS PubMed.
- H. M. Wang and Z. M. Yang, Molecular hydrogels of hydrophobic compounds: a novel self-delivery system for anti-cancer drugs, Soft Matter, 2012, 8, 2344–2347 RSC.
- W. Ha, J. Yu, X. Y. Song, J. Chen and Y. P. Shi, Tunable temperature-responsive supramolecular hydrogels formed by prodrugs as a codelivery system, ACS Appl. Mater. Interfaces, 2014, 6, 10623–10630 CAS.
- G. Wenz, B. H. Han and A. Muller, Cyclodextrin Rotaxanes and Polyrotaxanes, Chem. Rev., 2006, 106, 782–817 CrossRef CAS PubMed.
- F. H. Huang and H. W. Gibson, Polypseudorotaxanes and polyrotaxanes, Prog. Polym. Sci., 2005, 30, 982–1018 CrossRef CAS.
- A. Harada, Preparation and structures of supramolecules between cyclodextrins and polymers, Coord. Chem. Rev., 1996, 148, 115–133 CrossRef CAS.
- C. Hu and D. D. Kitts, Luteolin and Luteolin-7-O-glucoside from Dandelion Flower Suppress iNOS and COX-2 in RAW264.7 Cells, Mol. Cell. Biochem., 2004, 265, 107–113 CrossRef CAS PubMed.
- A. Harada, Design and construction of supramolecular architectures consisting of cyclodextrins and polymers, Adv. Polym. Sci., 1997, 133, 141–191 CrossRef CAS.
- Q. Zhang, L. Wan, Y. Guo, N. Cheng, W. Cheng, Q. Sun and J. Zhu, Radiosensitization effect of luteolin on human gastric cancer SGC-7901 cells, J. Biol. Regul. Homeostatic Agents, 2009, 23, 71–78 Search PubMed.
- A. K. Pandurangan, P. Dharmalingam, S. K. Anandasadagopan and S. Ganapasam, Effect of luteolin on the levels of glycoproteins during azoxymethane-induced colon carcinogenesis in mice, Asian Pacific Journal of Cancer Prevention: APJCP, 2012, 13, 1569–1573 CrossRef PubMed.
- S. H. Park, H. S. Park, J. H. Lee, G. Y. Chi, G. Y. Kim, S. K. Moon, Y. C. Chang, J. W. Hyun and Y. H. Choi, Induction of endoplasmic reticulum stress-mediated apoptosis and non-canonical autophagy by luteolin in NCI-H460 lung carcinoma cells, Food Chem. Toxicol., 2013, 56, 100–109 CrossRef CAS PubMed.
- Y. Jeon and Y. J. Suh, Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells, Oncol. Rep., 2013, 29, 819–825 CAS.
- C. Hu and D. D. Kitts, Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells, Mol. Cell. Biochem., 2004, 265, 107–113 CrossRef CAS PubMed.
- H. Veda, C. Yamazaki and M. Yamazaki, Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens, Biol. Pharm. Bull., 2002, 25, 1197–1202 Search PubMed.
- R. Liu, M. Gao, G. T. Qiang, L. X. Zhang, J. Ying and G. Du, The anti-amnesic effects of luteolin against amyloid β25–35 peptide-induced toxicity in mice involve the protection of neurovascular unit, Neuroscience, 2009, 162, 1232–1243 CrossRef CAS PubMed.
- K. Shimoia, H. Okadaa, M. Furugoria, T. Godaa, S. Takasea, M. Suzukib, Y. Harab, H. Yamamotoc and N. Kinaea, Intestinal absorption of luteolin and luteolin-7-O-β-glucoside in rats and humans, FEBS Lett., 1998, 438, 220–224 CrossRef.
- R. M. Xing, X. Y. Wang, C. L. Zhang, J. Z. Wang, Y. M. Zhang, Y. Song and Z. J. Guo, Superparamagnetic magnetite nanocrystal clusters as potential magnetic carriers for the delivery of platinum anticancer drugs, J. Mater. Chem., 2011, 21, 11142–11149 RSC.
- C. R. Heald, S. Stolnik, K. S. Kujawinski, C. Dematteis, M. C. Garnett, L. Illum, S. S. Davis, S. C. Purkiss, R. J. Barlow and P. R. Gellert, Poly(lacticacid)–poly(ethylene oxide) (PLA–PEG) nanoparticles: NMR studies of the central solid like PLA core and the liquid PEG corona, Langmuir, 2002, 18, 3669–3675 CrossRef CAS.
- X. B. Xiong, A. Mahmud and H. Uludag, Conjugation of arginine–glycine–aspartic acid peptides to poly(ethylene oxide)-b-poly(ε-caprolactone) micelles for enhanced intracellular drug delivery to metastatic tumor cells, Biomacromolecules, 2007, 8, 874–884 CrossRef CAS PubMed.
- A. Harada, J. Li and M. Kamachi, Double-stranded inclusion complexes of cyclodextrin threaded on poly(ethylene glycol), Nature, 1994, 370, 126–128 CrossRef CAS.
- A. Harada, Cyclodextrin-Based Molecular Machines, Acc. Chem. Res., 2001, 34, 456–464 CrossRef CAS PubMed.
- V. Gopishetty, I. Tokarev and S. Minko, Biocompatible stimuli-responsive hydrogel porous membranes via phase separation of polyvinyl alcohol and Na-alginate intermolecular complex, J. Mater. Chem., 2012, 22, 19482–19487 RSC.
- J. L. He, M. Z. Zhang and P. H. Ni, Rapidly in situ forming polyphosphoester-based hydrogels for injectable drug delivery carriers, Soft Matter, 2012, 8, 6033–6038 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20851g |
| This journal is © The Royal Society of Chemistry 2016 |