Highly stable electrical manipulation of reflective colors in colloidal crystals of sulfate iron oxide particles in organic media†
Abstract
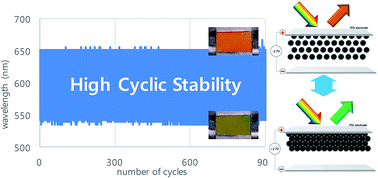
Monodisperse Fe3O4 particles are prepared by solvothermal reaction, in which negatively charged sulfate groups are formed on the surface by adding potassium sulfate during the high-pressure reaction. Then, those particles are successfully dispersed in the polar solvent propylene carbonate and manipulated electrophoretically between transparent electrodes. By applying an external electric field, they showed structural colors which can be tuned precisely over the visible range depending on the strength of the electric field. Because sulfate groups on the particle surface are chemically stable or inert, those particles are highly stable against irreversible deposition on the electrode during electrophoretic operation. Therefore, we demonstrate highly stable color switches over at least hundreds of cyclic operations, which may be useful in real electronic paper display applications.

 Please wait while we load your content...
Please wait while we load your content...