Four new silver phosphonates constructed from semi-rigid phosphonate ligands: synthesis, structure and properties†
Abstract
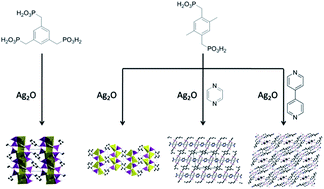
Four new silver phosphonates, namely, Ag3(H3L1) (1), Ag2(H2L2) (2), Ag2(H2L2)(pyz) (3) and Ag2(H4L2)(H2L2)(4,4′-bipy)(H2O) (4) (H6L1 = (benzene-1,3,5-triyltris(methylene))tris(phosphonic acid); H4L2 = (2,5-dimethyl-1,4-phenylene)bis(phosphonic acid); pyz = pyrazine; 4,4′-bipy = 4,4′-bipyridine) have been synthesized from the reactions of silver oxide (Ag2O) and tris or bisphosphonate ligands with or without the existence of second auxiliary ligands via hydrothermal methods. These silver phosphonates were systematically characterized using single-crystal and powder X-ray diffraction, IR, EA, TGA and fluorescence technologies. The phosphonate ligands adopt diverse coordination modes and coordinate to the silver ions with O–Ag bonds as well as C–Ag bonds, forming various coordination configurations around the silver ions. Compound 1 features a pillar-layered structure with silver and oxygen atoms as the layer and the organic linkers as pillars. Compound 2 displays a layer structure which can assemble into a three-dimensional supramolecular structure with the aid of O–H⋯O interactions. In compound 3, silver ions are linked into layers and further connected to form a pillar-layered structure with the pyrazine molecules as pillars. Compound 4 also displays a layer structure which is constructed from ladders and organic phosphonate ligands. The photophysical measurements suggested that these compounds all displayed intraligand fluorescence.

 Please wait while we load your content...
Please wait while we load your content...