Open Access Article
Open Access ArticleA closer look at two-photon absorption, absorption saturation and nonlinear refraction in gold nanoclusters
Joanna
Olesiak-Banska
*,
Magdalena
Waszkielewicz
,
Katarzyna
Matczyszyn
and
Marek
Samoc
Advanced Materials Engineering and Modelling Group, Faculty of Chemistry, Wroclaw University of Science and Technology, Wroclaw, Poland. E-mail: joanna.olesiak@pwr.edu.pl
First published on 12th October 2016
Abstract
The optical properties of atomically-precise gold nanoclusters differ significantly from those of plasmonic gold nanoparticles, in both linear and nonlinear regimes. Here, we present a systematic study on the third-order nonlinear optical (NLO) properties of thiol-protected gold clusters in a wide range of wavelengths. We applied the Z-scan technique to evaluate the wavelength dispersion of two-photon absorption (2PA) and nonlinear refraction of the nanoclusters in water. The results are discussed with reference to 2PA of plasmonic gold nanoparticles and literature data on NLO properties of gold nanoclusters.
Introduction
Gold nanoclusters (NCs) are a new class of metallic nanomaterial, which consist of a certain number of central metal atoms surrounded by different types of ligands. Because of their sizes (1–2 nm), nanoclusters present a quantum-size effect, which leads to a discrete electronic structure.1 The spectra of NCs exhibit molecular-like one-electron transitions rather than collective excitation like in plasmonic gold nanocrystals. The unique electronic and geometric structure of nanoclusters gives rise to interesting combinations of properties like chirality, magnetism, redox chemistry etc.2–4 Thus, nanoclusters have gained great interest and applications in catalysis, photonics, biosensing and molecular electronics.5,6The gold nanoclusters were shown to exhibit significant optical nonlinearities. Because of their molecule-like electronic level structures one can expect that their NLO behavior, in particular, their nonlinear refraction and absorption (including two-photon absorption, 2PA) properties should be understood in the same terms as the analogous properties of molecular materials (see e.g.ref. 7 and 8). Several reports on third-order NLO properties of gold nanoclusters have been published and we summarize their results in Table 1. Multiphoton absorption and emission of nanoclusters have been usually investigated at single wavelengths, which presents only a fragmentary image of the NLO properties. The reported two-photon absorption cross-section σ2 vary from several hundred GM to several hundred thousand GM. The discrepancies can be explained to some extent by various techniques and various measurement parameters applied. Especially, excitation with fs vs. ns laser pulses gives fundamentally different information about the NLO properties, as in the latter case cumulative effects may contribute significantly to the observed signal. Two-photon absorption (2PA)9 as well as saturable absorption (SA)10 were observed for gold nanoclusters under ns excitation in the one-photon absorption (1PA) range of wavelengths. On the other hand, measurements with fs pulses are usually based on two-photon excited fluorescence (2PEF), which provide the two-photon action cross section values Φσ2 (where Φ is the fluorescence quantum yield).11–13 The σ2 values calculated from those measurements vary significantly from those measured directly, e.g. by using the Z-scan technique.
| Reference | Materiala | Technique | Results |
|---|---|---|---|
| a Abbreviations: SR = SCH2CH2Ph, polyethylene glycol (PEG), mercaptoundecanoic acid (MUA), glutathione (SG), 1-dodecanethiol (DTT), 2-naphthalenethiol (NT), 4-methylbenzenethiol (mBT), α-toluenethiol (α-TT). | |||
| Philip et al.9 | Au25SR, Au38SR, Au144SR, Au 4 nm, in toluene | OA Z-scan, 5 ns, 532 nm (T = 25%), 15 μJ | 2PA: Au25: 2.0 × 10−10 m W−1, Au38: 3.5 × 10−10 m W−1, Au144: 7.5 × 10−10 m W−1 (I = 1.5 × 107 GW cm−2), Au 5 nm: 1.5 × 10−10 m W−1 (I = 2.2 × 107 GW cm2) |
| Ding et al.10 | AuNC in water | OA Z-scan, 4.8 ns, 532 nm, 5 kHz | SA: 0.029–0.07 GW cm2 for P = 2–3.7 mW |
| Ramakrishna et al.23 | Au25, Au140, Au309, Au976, Au2406 hexane | Fluorescence upconversion, 100 fs, 800 nm | 2PA: Au25: 427![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GM, Au140: 871 000 GM, Au140: 871![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GM, Au309: 1 000 GM, Au309: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 476![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GM, Au976: 905 000 GM, Au976: 905![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 GM, Au2406: 3 200 GM, Au2406: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 452 452![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GM 000 GM |
| Oh et al.12 | AuNC–PEG 1.5 nm ± 0.3 nm water | 2PEF, 850–1300 nm, ≈80 fs, 250 kHz | 2PA: decreasing from 670 GM at 870 nm, to 130 GM at 1300 nm |
| Liu et al.11 | Au11MUA 1.7 × 10−4 M, water | 2PEF 800 nm, MHz and OA Z-scan 800 nm, 1 kHz, 180 fs | 2PA: 3426 GM (800 nm, TPEF) 8761 GM (800 nm, OA Z-scan) |
| Russier-Antoine et al.13 | Au15SG Au25SG | 2PEF 800 nm, 140 fs, 76 MHz | 2PA: Au15 ∼ 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 GM (780 nm) 700 GM (780 nm) |
| Hamanaka et al.24 | Au25DDT, Au44DDT, Au66DDT, Au135DDT, Au247NT, Au376mBT, Au835α-TT, toluene, Au1800/SiO2 | Pump-probe differential absorption spectra, 430–775 nm, 150 fs, 1 kHz, <30 μJ mm−2 | 2PA and SA: Imχ3/α = 2 × 10−15 esu cm for Au25DTT decreasing to −8 × 10−15 esu cm for Au NP |
| This work | Au25Capt18, water | CA and OA Z-scan 550–1100 nm, 130 fs, 1 kHz, 35 to 180 GW cm−2 | 2PA and SA: σ2 = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 GM, 830 GM and 1510 GM at 550 nm, 800 nm and 900 nm 800 GM, 830 GM and 1510 GM at 550 nm, 800 nm and 900 nm |
We present here a systematic investigation of third-order NLO properties of gold nanoclusters by the Z-scan technique with fs laser excitation in a broad range of wavelengths. We chose to investigate thiol-protected Au25 nanoclusters as those have been most widely described and applied. We identify the NLO processes observed in the sample as 2PA and SA, depending on the incident laser wavelength. We present for the first time the dispersion of both nonlinear refraction and two-photon absorption of gold nanoclusters. Thus, we provide a full description of third order NLO properties. Finally, we can compare the results to those reported in the literature for gold nanoclusters as well as other gold nanoparticles.
Experimental
Nanoclusters synthesis
Gold-captopril nanoclusters (Au25Capt18) were prepared using a published procedure.14 Briefly, TOABr (0.23 mmol, 126.8 mg) was dissolved in 10 mL of methanolic solution of HAuCl4·3H2O (0.02 M) and after 20 min, 1 mmol of captopril (217.2 mg) was dissolved in 5 mL of methanol and rapidly added into the reaction mixture under stirring. After 30 min, 5 mL of aqueous solution of NaBH4 (0.4 M), cooled to 4 °C, was rapidly poured to the reaction mixture under vigorous stirring. The solution was allowed to react for 8 h in ice bath. The suspension was centrifuged. The clusters were repeatedly washed with methanol and ethanol. The final precipitate was dried in a water bath.Z-Scan measurements
The Z-scan measurements were performed as described previously, with some modifications.15 A Quantronix Integra-C regenerative amplifier operating as an 800 nm pump and a Quantronix-Palitra-FS optical parametric amplifier were used to deliver wavelength tunable pulses with the duration <130 fs, and a repetition rate of 1 kHz. The excitation beam with intensities ranging from 35 to 180 GW cm−2 was used for simultaneous recording of open aperture (OA) and closed-aperture (CA) Z-scan transmittance curves. Three Si photodiodes (Thor Laboratories Inc.) monitored the laser input, the OA signal, and the CA signal. An aqueous solution of nanoclusters (c = 11 mg mL−1) was placed in 1 mm path length Starna sealed glass cuvettes. A reference cuvette containing a pure solvent was always measured under identical conditions to enable extraction of nonlinear refraction of the nanoclusters from the overall response of the sample. No nonlinear absorption of the solvent was observed in the range of wavelengths and intensities employed.Results and discussion
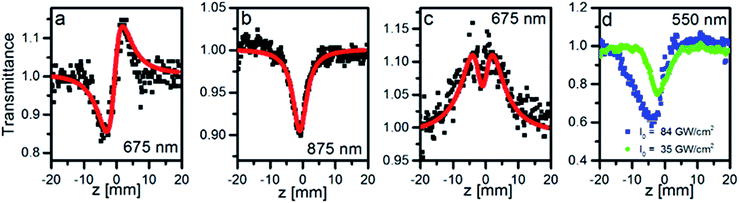
The 1PA spectrum of the studied nanoclusters Au25Capt18 presents the clearly resolved absorption bands in the NIR and visible range, which indicate that the synthesis yielded a monodisperse solution of NCs. The bands positions at 800 nm, 675 nm, 550 nm, (505 nm), 445 nm, (400 nm) correspond very well with theoretical and experimental spectra of Au25(SR)18 found in the literature (see Fig. 2, gray solid line).16We performed OA and CA Z-scan measurements on a water dispersion of Au25Capt18 in the range of wavelengths 550–1100 nm. Fig. 1 shows examples of OA and CA traces at several wavelengths. For all the wavelengths but 550 nm the CA traces present a decrease followed by an increase of transmittance, which is characteristic for a material with a positive (self-focusing) refractive nonlinearity (Fig. 1a). At 550 nm an opposite trend was observed, which implies overall negative refractive nonlinearity of the sample (including contributions from the cell walls, the solvent and the solute). OA traces showed a decrease of the transmittance in the range of wavelengths > 775 nm and <650 nm (Fig. 1b). However, an increase of the transmittance and combination of the increase followed by the decrease in the focus were observed when λ = 650–750 nm were applied (Fig. 1c). It has to be noted that the nanoclusters were sensitive to the high intensity illumination, especially when the laser wavelength falls into the range of wavelengths where 1PA is observed. Excessively high intensities resulted in a photochemical transformation in clusters, identifiable as a deformation of Z-scan traces: a characteristic asymmetry of the OA traces (Fig. 1d).
We analyzed the collected data using the equations derived by Sheik-Bahae et al.17 The nonlinear refractive index n2 and two-photon absorption coefficient α2 of the solutions were obtained from the nonlinear phase shift and the changes of transmittance of light passing through the sample. We calculated the NLO parameters of the solute assuming that the nonlinear contributions of the solvent and the solute sum up and the Lorentz local field approximation is valid.18
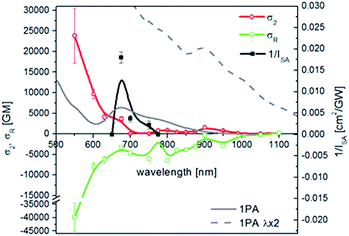
Two alternate ways of presenting the microscopic, concentration-independent cubic nonlinearities exist, either by quoting the real and imaginary parts of the second hyperpolarizability (third-order polarizability) of a species, Re[γ] and Im[γ], respectively, or by providing appropriate cross sections which are a measure of the nonlinear refraction and nonlinear absorption contributions. As proposed in ref. 19 we present here the spectra of σ2 of a single nanocluster and the cross-section characterizing the nonlinear refraction σR. Presentation of a single nanoparticle-oriented values of both refractive and absorptive part of the nonlinearity gives the basis for understanding NLO effects in gold nanoclusters. Also, it allows for quantitative comparison of NLO parameters between different types of nanoparticles and other materials. Fitting of the Z-scan traces led to σR and σ2 spectra presented in Fig. 2. The σR is negative in the whole range of wavelengths, with the magnitude increasing rapidly at shortest wavelengths up to −40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GM at 550 nm. 2PA bands at ∼800 and ∼900 nm are clearly visible, with σ2 = 850 and 1500 GM, respectively. These bands can be related to the d-sp interband transitions located at 400–450 nm in the 1PA spectrum.20 Additionally, in 550–650 nm range a strong 2PA was observed with σ2 increasing up to 24
000 GM at 550 nm. 2PA bands at ∼800 and ∼900 nm are clearly visible, with σ2 = 850 and 1500 GM, respectively. These bands can be related to the d-sp interband transitions located at 400–450 nm in the 1PA spectrum.20 Additionally, in 550–650 nm range a strong 2PA was observed with σ2 increasing up to 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GM. Resonant excitation in the range of 1PA implies possible excited state absorption mechanism of this transition.
000 GM. Resonant excitation in the range of 1PA implies possible excited state absorption mechanism of this transition.
To reproduce the increase of the transmittance observed in OA Z-scans for λ = 650–750 nm we had to introduce to our calculations an absorption saturation effect. Several models of SA in metal nanoparticles has been reported, including 1PA saturation and 2PA.10,15,21 In case of gold nanoclusters, they present complex 1PA spectra with mixed contributions of core and surface states.20 A simplified three-level energy diagram can be applied, which consists of a ground state S0, the first and second excited states S1 and S2, respectively, (as Au25Capt18 nanoclusters exhibit long photoluminescence decay times, on the order of a few μs (ref. 22) triplet states may be in fact involved). Relaxation from the higher-lying singlet and triplet states is rapid, thus the population densities of these states are very small and can be ignored.
The rate equation of the excited state S1 and the equation describing changes of the absorption of the incident light are:
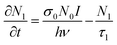 | (1) |
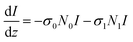 | (2) |
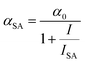 | (3) |
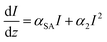 | (4) |
The formula accounts for an interplay between SA and 2PA, which, when present simultaneously, result in the OA trace, where a decrease of T at the focus of the beam is superimposed on the broader increase. Eqn (4) was applied for fitting OA traces at λ = 650–750 nm and good reproduction of experimental results was obtained. The calculated 1/ISA values are presented in Fig. 2.
Table 1 shows the NLO coefficients for Au NCs reported in the literature and the present results. Experiments with fs pulsed lasers (usually 2PEF) gave σ2 values ranging from 427![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GM down to 670 GM at ∼800 nm. On the other hand, when the Z-scan technique with ns lasers was applied, contradicting results were reported for the same wavelength, either 2PA or SA.9,10 No σ2 values were calculated, and α2 was determined at 532 nm to be from 2.0 × 10−10 m W−1 for Au25 up to 7.5 × 10−10 m W−1 for Au144. Liu et al. compared σ2 measured with Z-scan and 2PEF methods and obtained values of 8761 GM and 3426 GM, respectively.11 Note that the present report is the first to provide the full, wide-wavelength-range spectral characterization of the NLO properties of the NCs.
000 GM down to 670 GM at ∼800 nm. On the other hand, when the Z-scan technique with ns lasers was applied, contradicting results were reported for the same wavelength, either 2PA or SA.9,10 No σ2 values were calculated, and α2 was determined at 532 nm to be from 2.0 × 10−10 m W−1 for Au25 up to 7.5 × 10−10 m W−1 for Au144. Liu et al. compared σ2 measured with Z-scan and 2PEF methods and obtained values of 8761 GM and 3426 GM, respectively.11 Note that the present report is the first to provide the full, wide-wavelength-range spectral characterization of the NLO properties of the NCs.
In line with our previous results, we can determine certain figures of merit for the NLO properties of NCs and compare them with those for other gold nanoparticles. A convenient figure of merit is σ2 scaled by the object molar mass. For gold nanorods (aspect ratio 3.4, λl-SPR = 845 nm, σ2 at 550 nm) and gold nanoshells (120 nm silica nanospheres with ∼10 nm gold shell, σ2 at 600 nm) we obtained values of 7.96 and 2.56, respectively (in GM g mol−1).15,21,25 In comparison, for Au25(Capt)18 at 550 nm we measured σ2/Mw = 2.69, thus the NCs are good two-photon absorbers, comparable to gold nanoshells, although they contain a significantly lower gold content. In comparison with the best two-photon absorbing organic molecules, nanoclusters exhibit significantly higher σ2 and σ2/Mw values (e.g. for an N-cored Ru-containing dendrimer, σ2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 GM and σ2/Mw = 1.13 at 580 nm).26
600 GM and σ2/Mw = 1.13 at 580 nm).26
Conclusions
In summary, we measured for the first time the full two-photon absorption spectrum and dispersion of nonlinear refraction of thiol-stabilized gold nanoclusters in water. Very strong 2PA was seen in the range of 1PA wavelengths, with σ2 up to 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GM. Other 2PA bands of lower intensity at 800 and 900 nm were characterized by cross sections of 830 GM and 1510 GM, respectively. Nonlinear refraction cross-section values were negative in the whole range of wavelengths. Moreover, SA effects were found to dominate when the incident wavelength was from 650 to 750 nm. Efficient 2PA makes gold nanoclusters good candidates for optical limiting applications, and, combined with their fluorescent properties, a marker for multiphoton imaging.
000 GM. Other 2PA bands of lower intensity at 800 and 900 nm were characterized by cross sections of 830 GM and 1510 GM, respectively. Nonlinear refraction cross-section values were negative in the whole range of wavelengths. Moreover, SA effects were found to dominate when the incident wavelength was from 650 to 750 nm. Efficient 2PA makes gold nanoclusters good candidates for optical limiting applications, and, combined with their fluorescent properties, a marker for multiphoton imaging.
Acknowledgements
This work was supported by the National Science Centre under grant DEC-2013/10/A/ST4/00114, DEC-2013/09/B/ST5/03417 and by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wroclaw University of Technology.References
- R. C. Jin, Nanoscale, 2010, 2, 343 RSC
.
- S. Knoppe and T. Burgi, Acc. Chem. Res., 2014, 47, 1318 CrossRef CAS PubMed
.
- W. Luo, S. J. Pennycook and S. T. Pantelides, Nano Lett., 2007, 7, 3134 CrossRef CAS PubMed
.
- H. Tsunoyama, H. Sakurai, Y. Negishi and T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 9374 CrossRef CAS PubMed
.
- P. Schwerdtfeger, Angew. Chem., Int. Ed., 2003, 42, 1892 CrossRef CAS PubMed
.
- L. Shang, S. Dong and G. U. Nienhaus, Nano Today, 2011, 6, 401 CrossRef CAS
.
- M. G. Kuzyk, K. D. Singer and G. I. Stegeman, Adv. Opt. Photonics, 2013, 5, 4 CrossRef
.
- F. Terenziani, C. Katan, E. Badaeva, S. Tretiak and M. Blanchard-Desce, Adv. Mater., 2008, 20, 4641 CrossRef CAS
.
- R. Philip, P. Chantharasupawong, H. F. Qian, R. C. Jin and J. Thomas, Nano Lett., 2012, 12, 4661 CrossRef CAS PubMed
.
- S. J. Ding, F. Nan, D. J. Yang, X. L. Liu, Y. L. Wang, L. Zhou, Z. H. Hao and Q. Q. Wang, Sci. Rep., 2015, 5, 9735 CrossRef CAS PubMed
.
- C. L. Liu, M. L. Ho, Y. C. Chen, C. C. Hsieh, Y. C. Lin, Y. H. Wang, M. J. Yang, H. S. Duan, B. S. Chen, J. F. Lee, J. K. Hsiao and P. T. Chou, J. Phys. Chem. C, 2009, 113, 21082 CAS
.
- E. Oh, F. K. Fatemi, M. Currie, J. B. Delehanty, T. Pons, A. Fragola, S. Lévêque-Fort, R. Goswami, K. Susumu, A. L. Huston and I. L. Medintz, Part. Part. Syst. Charact., 2013, 30, 453 CrossRef CAS
.
- I. Russier-Antoine, F. Bertorelle, M. Vojkovic, D. Rayane, E. Salmon, C. Jonin, P. Dugourd, R. Antoine and P. F. Brevet, Nanoscale, 2014, 6, 13572 RSC
.
- S. Kumar and R. Jin, Nanoscale, 2012, 4, 4222 RSC
.
- J. Olesiak-Banska, M. Gordel, R. Kolkowski, K. Matczyszyn and M. Samoc, J. Phys. Chem. C, 2012, 116, 13731 CAS
.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883 CrossRef CAS PubMed
.
-
E. W. V. Stryland and M. Sheik-Bahae, Z-Scan measurements of optical nonlinearities, in Characterization techniques and tabulations for organic nonlinear optical materials, ed. M. G. Kuzyk and C. W. Dirk, Marcel Dekker, New York, 1998, pp. 655–692 Search PubMed
.
- M. Samoc, A. Samoc, B. Luther-Davies, M. G. Humphrey and M. S. Wong, Opt. Mater., 2003, 21, 485 CrossRef CAS
.
- M. Balu, L. A. Padilha, D. J. Hagan, E. W. Van Stryland, S. Yao, K. Belfield, S. Zheng, S. Barlow and S. Marder, J. Opt. Soc. Am. B, 2008, 25, 159 CrossRef CAS
.
- C. M. Aikens, J. Phys. Chem. Lett., 2011, 2, 99 CrossRef CAS PubMed
.
- M. Gordel, J. Olesiak-Banska, R. Kolkowski, K. Matczyszyn, M. Buckle and M. Samoc, J. Mater. Chem. C, 2014, 2, 7239 RSC
.
- Z. K. Wu and R. C. Jin, Nano Lett., 2010, 10, 2568 CrossRef CAS PubMed
.
- G. Ramakrishna, O. Varnavski, J. Kim, D. Lee and T. Goodson, J. Am. Chem. Soc., 2008, 130, 5032 CrossRef CAS PubMed
.
- Y. Hamanaka, N. Okada, K. Fukagawa, A. Nakamura, Y. Tai and J. Murakami, J. Phys. Chem. C, 2012, 116, 10760 CAS
.
- M. Gordel, R. Kolkowski, J. Olesiak-Banska, K. Matczyszyn, M. Buckle and M. Samoć, J. Nanophotonics, 2014, 9, 093797 CrossRef
.
- R. L. Roberts, T. Schwich, T. C. Corkery, M. P. Cifuentes, K. A. Green, J. D. Farmer, P. J. Low, T. B. Marder, M. Samoc and M. G. Humphrey, Adv. Mater., 2009, 21, 2318 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |