Open Access Article
Open Access ArticlePectinases immobilization on magnetic nanoparticles and their anti-fouling performance in a biocatalytic membrane reactor†
Abaynesh Yihdego
Gebreyohannes
 a,
Rosalinda
Mazzei
a,
Teresa
Poerio
a,
Pierre
Aimar
b,
Ivo F. J.
Vankelecom
c and
Lidietta
Giorno
a,
Rosalinda
Mazzei
a,
Teresa
Poerio
a,
Pierre
Aimar
b,
Ivo F. J.
Vankelecom
c and
Lidietta
Giorno
 *a
*a
aInstitute on Membrane Technology ITM-CNR, National Research Council of Italy, Via P. Bucci, CUBO 17/C UNICAL Campus, 87036 Rende (CS), Italy. E-mail: abayneshy@yahoo.com; a.gebreyohannes@itm.cnr.it; l.giorno@itm.cnr.it; r.mazzei@itm.cnr.it; t.poerio@itm.cnr.it
bLaboratoire de Génie Chimique, Université de Toulouse, CNRS, INPT, UPS, Toulouse, France. E-mail: Pierre.Aimar@univ-toulouse.fr
cCentrum voor Oppervlaktechemie en Katalyse Dept. M2S, Faculteit Bio-ingenieurswetenschappen, KU Leuven, Leuven Chem & Tech, Postbus 2461, Celestijnenlaan 200F, 3001 Leuven, Belgium. E-mail: ivo.vankelecom@biw.kuleuven.be
First published on 10th October 2016
Abstract
Enzyme immobilization on commercial superparamagnetic nanoparticles (NPSP) was performed using covalent bonding. The biofunctionalized NPSP was then immobilized on the surface of the membrane using an external magnetic field to form a magneto-responsive biocatalytic membrane reactor (BMRSP). The magnetically formed smart nanolayer can be easily re-dispersed and recovered from the membrane when the enzyme is deactivated or whenever cleaning is required due to substrate over-accumulation. The system was used to hydrolyze pectin contained in different streams. Results are supported with complementary data from hydrodynamic, kinetic and morphological characterization in a flow-through reactive filtration. Wavelength-dispersive X-ray spectroscopy (WDS) elemental mapping revealed that the NPSP are uniformly dispersed on the surface of the membrane forming a thin biocatalytic layer. Both results of hydrodynamic studies and SEM micrographs of the membrane with the enzyme layer under various operating conditions, show that the immobilized enzyme effectively reduced membrane–foulant interaction. Comparison of filtration data using this commercial NPSP reveals good agreement with our previously used home-made NPSP. This implies that the scaling-up and commercialization of the developed BMRSP can be straightforward.
1. Introduction
Bionanocomposites represent an emerging group of bioinspired and biomimetic nanostructured hybrid materials that take the advantage of organic and inorganic moieties.1 They are formed by the hybridization of natural polymers and inorganic solids e.g., iron based magnetic particles on the nanometer scale. The bionanocomposites are thus combination of synthetic and biological polymers. Hence it involves coating inorganic particles with synthetic polymers to introduce surface functional groups which are subsequently used for the anchorage of biopolymers.2 The combined biohybrid materials with its inherent biocompatibility, biodegradability and high-surface area-to-volume ratio exhibit improved structural and functional properties of great interest for various applications.3Bionanocomposites bearing functional biomolecules, like enzymes, take inspiration from nature to develop new hybrid biomaterials that mimic the exceptional features of natural nanocomposites. In superparamagnetic nanoparticle (NPSP) based bionanocomposites, the inorganic counterpart is mainly envisaged as a stimulus-responsive hybrid system in addition to its common supporting feature to preserve the immobilized biomolecules from denaturation.4,5
On the other hand, biocatalyst immobilized on nano-sized particles e.g., titania, carbon nanotubes with maximum surface area and activity have been applied in biocatalysis.6–9 The key drawback of such stirred tank reactors (STR) with inert carrier particles is recovery of the nano-catalyst, enzyme-product inhibition and regeneration or disposal of spent materials.10 An alternative is to coat the biocatalyst on polymeric membranes in a biocatalytic membrane reactor (BMR).11 This creates reactive surfaces for enhanced separations while eliminating the complexity of biocatalyst recovery. However, this method mainly suffers from irreversible attachment of the biocatalyst on the membrane and loss in intrinsic permselectivity of the membrane.12 In particular, enzyme deactivation during membrane cleaning is among the current bottle-necks that limit the speed-up of BMR at industrial level.
Therefore, the assembly of enzymes on magneto-responsive nanocomposites hosts a suitable alternative to the common methods used to immobilize enzymes directly on membranes. The immobilization of enzymes on magneto-responsive nanocomposites may result in robust hybrid materials, useful for the development of innovative stimuli-responsive BMRs able to extend the life time of both membrane and enzyme. In particular, the NPSP with immobilized enzyme can be easily attracted towards the surface of a membrane using an external magnetic field. This reversible deposition of the enzyme on the membrane facilitates the removal of the enzyme before membrane cleaning, thus avoiding enzyme denaturation due to change in the membrane microenvironment that could be imposed by membrane cleaning conditions.
The research on NPSP based stimuli-responsive BMR can be regarded as a new interdisciplinary field closely related to significant topics such as biomineralization processes, bioinspired materials, biomimetic systems and bio-separations. The upcoming development of novel bionanocomposite based enzyme immobilization introducing multi-functionality thus represents a promising research topic in the development of advanced BMRs for versatile applications such as environmental remediation.
The present work focuses on the immobilization of pectinase, used as “model enzyme” on NPSP to improve the stability of the enzymes and enhance their recovery by exploiting the properties of NPSP to be easily retrieved by application of an external magnetic field.
The presence of the NPSP on the surface of the membrane prevents direct substrate–membrane interactions, hence avoiding irreversible membrane fouling. There is also efficient in situ hydrolysis of substrate at the membrane–solution interface, thanks to the hydrolytic effect of the enzyme immobilized on the NPSP. Subsequently, easy removal of the dynamic layer of the enzymatically activated NPSP and easy recovery of both nanocatalyst and membrane is achievable. Hence, the present work demonstrates that our proposed system has the unique advantage of combining excellent antifouling properties of a BMR to the possibility to detach on demand the immobilized enzyme from the membrane during cleaning.
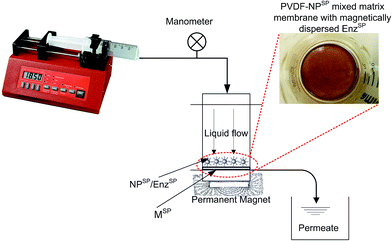
Since the dynamic layer of NPSP is formed using a nano-sized particle, a high pressure drop and increased mass transfer resistance is theoretically expected. However, the system exhibited no filtration resistance due to presence of the NPSP dynamic layer. This is a great improvement to the direct integration of enzyme into polymeric membranes, which mostly suffers from reduced intrinsic membrane permselectivity. This phenomenon is due to the alignment of the NPSP along an external magnetic field, which is applied parallel to the surface of magneto-responsive membrane. The magneto-responsive membrane, i.e. a membrane that contains NPSP within the polymer matrix, acted as a magnetic field actuator which ensured the even distribution and dispersion of NPSP over the surface of the membrane (see inset of Fig. 1).
In this work, process performance, fouling, morphological characterization and kinetic properties biofunctionalized membranes have been investigated demonstrating the upgrading of this innovative system from a basic proof-of-concept to a validation in laboratory environment.
The performance and reproducibility of commercially available NPSP are investigated instead of using home-made NPSP. Since the commercial NPSP exhibit a different particle size than what has already been used, it could also be used to overlay better understanding on the effect of particle size on the overall performance.
First, the immobilization capacity of a commercially available NPSP using enzyme pectinase is studied. Pectinase is a pectolytic enzyme capable of catalyzing the hydrolysis of glycoside bonds present in the structure of pectin, producing galacturonic acid (GalA) as its final product. Pectin are plant materials present in fruit juices, wine and vegetation wastewater. They are responsible for the formation of membrane fouling since they are gel forming agents. They thus form an impermeable layer on the membrane surface that subsequently reduces membrane performance. The activity of the enzyme was evaluated using the formation of GalA. Detailed characterization of the system through dynamic light scattering (DLS), thermo-gravimetric analysis (TGA), scanning electron microscopy (SEM) in backscattered detector (BSE) mode and wavelength-dispersive X-ray spectroscopy (WDS) is carried out. The capacity of immobilized pectinase to enhance surface biocatalysis in a continuous flow biocatalytic membrane reactor (BMRSP) is illustrated. The performance of the BMRSP is studied by varying substrate concentration, flux, and amount of NPSP. Subsequently, foulant deposition on the surface of the membrane in the presence and absence of biofunctionalized NPSP was morphologically characterized.
2. Materials and methods
2.1. Materials
Citrus fruit pectin (25–35% degree of esterification), polygalacturonase, galacturonic acid, 2-cyanoacetamide (99%) and protein bicinchoninic acid (BCA) were obtained from Sigma-Aldrich (Italy); borate buffer solution (pH 9.2) was from Fisher scientific. A 1% superparamagnetic nanoparticle dispersion (NPSP), EGDE (ethylene glycol diglycidyl ether MW = 174.2) and an activation buffer were acquired from ADEMTECH (Li StarFish s.r.l., Milan, Italy).The covalent immobilization of enzymes on the NPSP occurs by the functionalization of the polymer coated NPSP with carboxyl groups through which the links with the amino groups of the enzyme are established. Prior to enzyme immobilization, the NPSP was activated with EGDE (ethylene glycol diglycidyl ether MW = 174.2) and an activation buffer (patented ADEMTECH, Li StarFish s.r.l., Milan, Italy) to introduce carboxylic group to the surface of the NPSP. The carboxylic group eventually helped to create the covalent bond between the enzyme and the NPSP.
2.2. Activation of the NPSP
A three step procedure was followed to introduce the surface functional groups onto the polymer coated NPSP. The first phase consists in treating 3 mL of NPSP (1%) with 1% activation buffer. The second stage involves the addition of 100 μL EGDE per mg of NPSP followed by a thorough homogenization of the suspension. Finally the suspension was incubated for 2 h at 20 °C and maintained under constant mechanical stirring at 200 rpm. Ultimately, the NPSP activated with EGDE were washed twice with 1% activation buffer to avoid none-covalently attached EGDE.2.3. Enzyme immobilization
For the immobilization of pectinase, enzyme solution was prepared by dissolving pectinase powder in a 100 mM acetate buffer at pH 4.5. The mixture was left to solubilize by magnetically stirring over 24 h.The prepared solution containing 1.7 mg of pectinase was put in contact with –COOH functionalized NPSP in duplicate and kept under mechanical stirring for 2 h at ambient temperature, inorder to covalently attach the enzyme to the surface of the NPSP.
At the end of immobilization procedures, the enzymatically functionalized NPSP (EnzSP) were separated from the reaction mixture by using an external permanent magnet. The magnetically isolated supernatant was kept at 4 °C for indirect quantification of the amount of immobilized enzyme, by mass balance. Subsequently, the particles were washed trice with ultrapure water. The three step wash water was spectrophotometrically analyzed at a wavelength of 280 nm to assess the possible presence of protein leached during the washing.
To quantify the amount of immobilized enzyme, the immobilization solution before and after immobilization was measured by the BCA assay. The amount of immobilized enzyme was then calculated as:
| Massimmob. = massinitial soln − massfinal soln − masswashing soln |
Alternatively, a known amount of pectinase immobilized EnzSP was injected directly in the BCA reaction medium for quantification of the amount of enzyme immobilized on the surface of the NPSP.
2.4. Measurement of enzyme activity
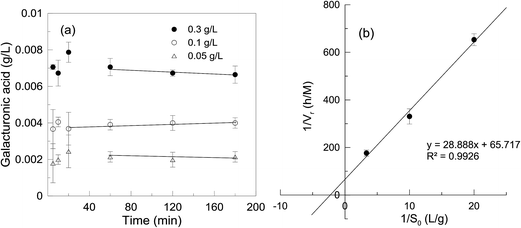
The activity of the immobilized enzyme in a STR and flow-through MF was evaluated by measuring the rate of product formation.During the activity measurement, the concentration of the substrate must always be in excess of the enzyme in order to saturate the enzyme completely. For enzymes that obey the Michaelis–Menten kinetics, a substrate concentration equal to ten times the Km is sufficient to bring the reaction rate very close to the Vmax. Hence the concentration of pectin in the STR was set at 4 g L−1, i.e. about ten times more than the Km value (0.5 g L−1) obtained for free enzyme in our previous work.13 The solution was kept under stirring for about 30 min at 40 °C prior to adding the EnzSP.
The method of detecting the GalA is based on the Knoevenagel condensation reaction between the active methyl group of cyanoacetamide and the aldehyde group of GalA to form a colored product.14 Hence, at the end of the reaction time, 1 mL of hydrolysate withdrawn from the reaction mixture was added to 2 mL of cold borate buffer to instantaneously terminate the reaction. After adding 1 mL of 1% 2-cyanoacetamide, the vortex mixed mixture was heated to 100 °C in an oil bath for 10 minutes. The absorbance of the sample, after cooling to room temperature, was measured at a wavelength of 274 nm against ultrapure water. Results were normalized with background GalA concentrations that were present in the feed solution prior to the enzymatic hydrolysis.15 The concentration of GalA was calculated from the standard curve obtained using solutions of GalA at different concentrations (0.01 to 0.3 g L−1). The enzyme activity is defined as the amount of GalA produced per min (μmol min−1) at 40 °C and pH 4.5.
2.5. Experimental set-up
The efficacy of the EnzSP for the biocatalytic reduction of fouling was evaluated through an MF system (Fig. 1) consisting of a syringe pump (New Era Pump Systems Inc), plastic syringe with 100 mL capacity, a digital manometer (WIKA corporate), a metallic part-free dead-end membrane module (Amicon cell), and a permanent magnet. The filtration cell is immersed in a thermo regulated water bath in order to have better control over the filtration temperature. The membrane used for filtration was lab-made magnetic responsive polyvinylidene fluoride (PVDF) membrane. It is basically a mixed matrix membrane prepared by mixing a 0.33 w/w% home-made NPSP that has an average particle size of 8 nm. The membrane has an average pore size of 0.1 μm. Further details on the membrane properties are provided in our previous work.12The membrane was tested by the following operating conditions: from 0.01 g L−1 to 1 g L−1 of pectin concentration, from 1 to 9 g m−2 of EnzSP amount per membrane area, flux ranging from 15 to 45 L m−2 h−1 and filtration temperature to 40 °C. The system performance was evaluated by measuring the change in transmembrane pressure (TMP) required to keep the flux constant in a continuous flow BMRSP.
2.6. Membrane and NPSP characterization
The NPSP size and size distribution before and after enzyme immobilization was determined by dynamic light scattering (DLS) (Mastersizer 2000, Malvern Instruments) in sodium acetate buffer at pH 4.5 (a buffer suitable for enzyme pectinase) after sonicating a diluted sample to avoid particles aggregation. Thermo-gravimetric analysis (TGA) was conducted using a Perkin-Elmer TGA 6 at a constant heating rate of 5 °C min−1 from room temperature to 500 °C in a N2 environment.To study the topography and distribution of the magnetically deposited EnzSP, cross-section of the EnzSP-loaded membranes that was used under various operating conditions were observed by means of scanning electron microscopy (SEM) images, backscattered electron images (BSE) and wavelength dispersive X-ray spectroscopy (WDS) elemental maps with a high resolution SEM (FEI QUANTA 200 SEM). Membrane samples were broken in liquid nitrogen to keep the film structure unaltered. SEM analyses were also carried out, after air drying of a dispersion of NPSP on a carbon tape.
3. Results and discussion
3.1. NPSP characterization
Fig. 2a represents SEM micrographs of the NPSP, which hold a monodispersed particle size. In agreement with the information provided by the supplying company, DLS analysis (Fig. S1a†) indicated an average particle size of 200 nm with polydispersity index (pdi) of 0.105. As expected, Fig. 2b shows that the NPSP (EnzSP) exhibited a slightly higher particle size due to biofunctionalization (216–250 nm) with 0.06 pdi (Fig. S1b†). Moreover, the EnzSP which were well dispersed in the liquid medium responded well to the applied magnetic field, by getting preferentially attached to the point of high magnetic field gradient (see inset of Fig. S2†).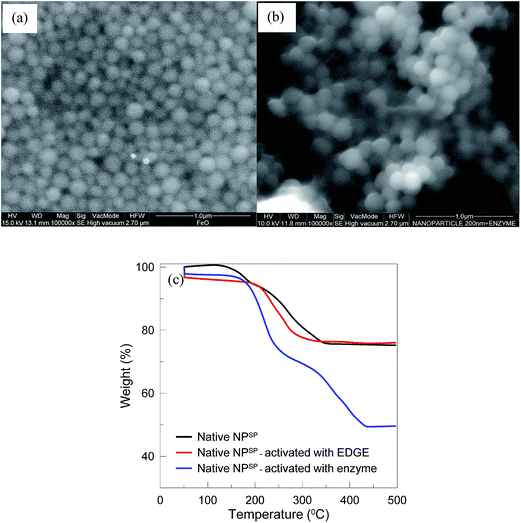 | ||
| Fig. 2 Characterization of NPSP before and after enzyme immobilization (a) SEM of NPSP before immobilization; (b) SEM of NPSP after pectinase immobilization; (c) TGA profile of NPSP. | ||
TGA curves show single transitions for both native and EDGE modified NPSP samples between 200 °C and 350 °C (Fig. 2c). This desorption is explained by the decomposition of the polymer shell used to coat the NPSP. For the EnzSP however, there were two-step desorption processes that occurred in the vicinity of 200 °C and 300 °C and 300 °C to 450 °C. The two step desorption processes were explained by the quasi-two-layers adsorbed models on the particle surface.16 The first one, which corresponds to a 30% weight loss, was due to the decomposition of the immobilized enzyme, while the second one, which corresponds to a 20% weight loss, was due to the polymer coating layer.
3.2. Magnetic dispersion of EnzSP the on membrane surface
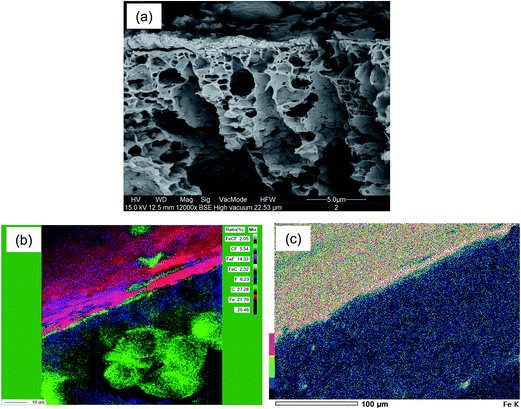
Surface and cross-sectional WDS maps of the membrane layered with 3 g m−2 EnzSP are shown in Fig. 3. Fig. 3a shows SEM images of the cross-section view of the membrane that contained 3 g m−2 EnzSP prior to the WDS elemental mapping. The images of the chemical elemental maps, acquired by WDS, show the spatial distribution of elements in the analyzed samples. The different concentrations of each element are shown by the color coding. By this mapping, it is possible to observe the uniform dispersion of the EnzSP over the surface of the membrane. High intensities, as represented by reddish/pinkish color (Fig. 3b), confirm the presence of Fe-rich dynamic layer. The area that shows green patches was most likely due to peeling-off the dynamic layer during sample drying and preparation for WDS.Fig. 3c is the single element map of Fe along the membrane cross-section containing 3 g m−2 EnzSP after filtering 0.3 g L−1 pectin solution at 15 L m−2 h−1. The higher concentration of the EnzSP on the top surface can be clearly recognized by its reddish/yellowish color in the map. The mixture of various colors shown in the map is attributed to the presence of raw or partially hydrolyzed pectin that could interfere with the detection of Fe. The membrane cross-section below the dynamic layer revealed a blue color related to low Fe concentration in the shown color coding.
3.3. Application of pectinase functionalized NPSP in a membrane bioreactor
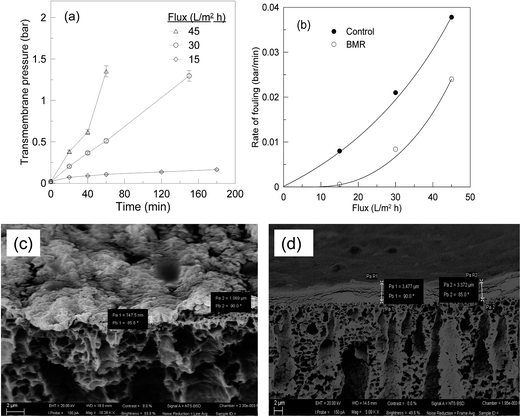
The EnzSP was used in a BMRSP for the hydrolysis of pectin contained in different streams. The effect of membrane fouling due to adsorbed pectin in the BMRSP was evaluated by measuring the change in TMP required to keep the flux constant in a constant volume and constant flow MF.As shown in Fig. 4, two systems, one containing a 3 g m−2 dynamic layer of neutral NPSP and another containing a 3 g m−2 immobilized pectinase containing EnzSP, were run in parallel. In both cases, the dynamic layer could prevent direct membrane–foulant interaction, hence avoiding a possible future membrane replacement due to irreversible fouling formation. When the EnzSP was used, the system faced limited effect of fouling thanks to the hydrolytic action exerted by the EnzSP on the adsorbed pectin.
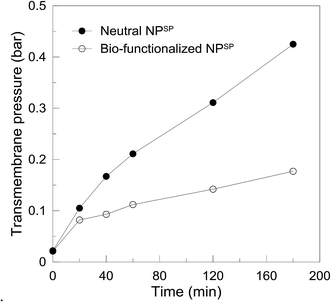 | ||
| Fig. 4 Transmembrane pressure as a function of time obtained using 3 g m−2 neutral NPSP and 3 g m−2 EnzSP at 15 L m−2 h−1 constant flux, 0.3 g L−1 pectin solution and 40 °C in a continuous flow MF. | ||
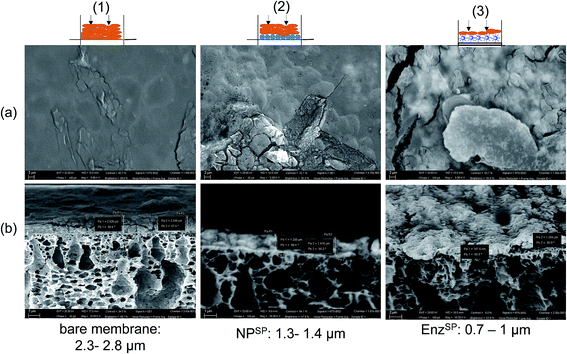
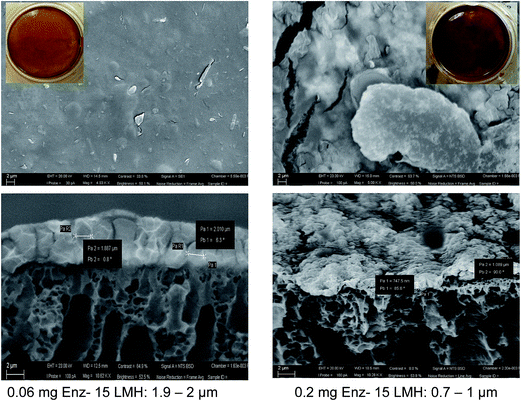
In Fig. 5 SEM micrographs of membranes characterized after the filtration of 0.3 g L−1 of pectin solution using a constant flux of 15 L m−2 h−1 is shown. The first column (Fig. 5(1a and b)) represents the magnetic responsive bare membrane after pectin filtration. The membrane was fully covered with the foulant while the cross-sectional image revealed a 3 μm thick foulant layer. In the second column (Fig. 5(2a and b)), before the fouling experiment, a dynamic layer 3 g m−2 neutral NPSP was magnetically attracted to the membrane surface while the third one contained similar amount of EnzSP. It is worth noting that due to the in situ degradation of the pectin by the immobilized pectinase, the thickness of the EnzSP (Fig. 5(3a and b)) plus the foulant layer were 30% and 67% lower than the total thickness observed for membranes containing neutral NPSP layer or no particles at all respectively.
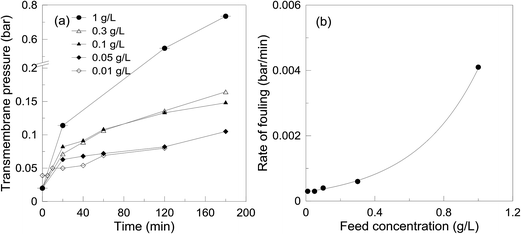 | ||
| Fig. 6 Effect of feed concentration on the filtration performance at 15 L m−2 h−1 constant flux, 3 g m−2 EnzSP and 40 °C; (a) TMP trend and (b) rate of fouling. | ||
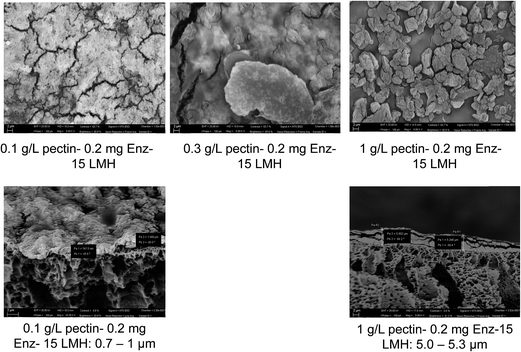
In Fig. 7, surface and cross-sectional SEM micrographs of the membranes used in the different feed concentrations are depicted. Similar to the TMP trend observed in Fig. 6, the total thickness of (foulant + EnzSP) layer increased with increased substrate concentration. Nevertheless, thanks to the hydrolytic effect of the EnzSP, when the feed concentration was raised tenfold, there only was a fivefold increase (from 1 μm to 5 μm) in the thickness of the layer. From the SEM surface images, a significantly different morphological structure of the foulant layer under different feed concentration can also be observed. In particular, the membrane used with the 1 g L−1 feed revealed the formation of bigger aggregates of foulants. These aggregates might be the result of a thick layer of foulant formed on the surface of the biocatalytic layer that eventually induced bigger aggregate formation upon drying the sample in the process of making it ready for the SEM analysis.
Moreover, all SEM micrographs are taken in backscattered detection (BSE) mode. BSE images display atomic number contrast with brighter regions being generated from areas of higher average atomic number. The surface micrographs showed a very bright image when low feed concentration (0.1 g L−1) was used. This is due to better visibility of iron contained in the EnzSP layer as there was only a very thin layer of foulants. However, with increased feed concentration and the subsequent thick layer of foulant, the bright color started to fade. These color patterns were also in good agreement with the observed TMP trend.
In the current system, in order to increase the amount of enzyme on the surface of the membrane, the amount of EnzSP was increased. By increasing the amount of EnzSP, it was possible to pack a multilayer of enzyme with a better distribution i.e. increase the surface area for enzyme immobilization over a given membrane area. As a result, the TMP required to keep the flux constant at 15 L m−2 h−1 decreased significantly while increasing the amount of EnzSP deposited per membrane area (Fig. 9a). The rate of fouling indicated in Fig. 9b also showed a logarithmic reduction. Hence, it resolved the issue of limited surface area for enzyme immobilization.
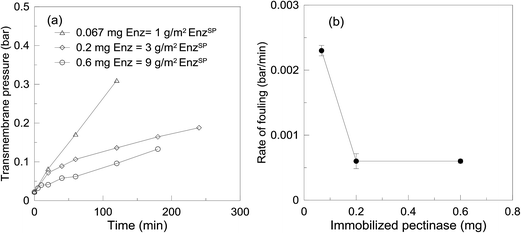 | ||
| Fig. 9 TMP as a function of time (a) and rate of fouling (b) obtained using different amount of pectinase loaded NPSP. | ||
Looking at the SEM images in Fig. 10, a threefold increase in the amount of EnzSP, resulted in a twofold reduction of the total thickness. Since the amount of EnzSP at the lowest enzyme concentration is significantly low, the relatively thicker layer of deposits observed at the lowest EnzSP was mainly due to membrane foulants.
In agreement with the TMP trend, when the flux was increased from 15 to 45 L m−2 h−1, where mass transfer rate prevails reaction rate, the thickness of the deposit on the membrane increased from 1 μm (Fig. 11c; left) to 3 μm (Fig. 11d; right).
3.4. Performance comparison between lab-made and commercial NPSP
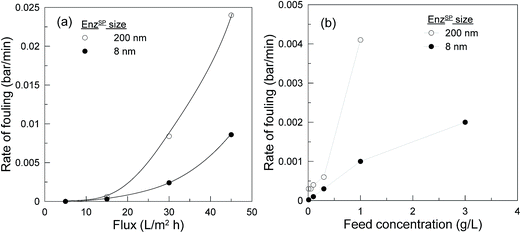
The performance and reproducibility of commercially available NPSP are compared with our previously used home-made NPSP with the aim to upgrade this innovative system from a basic proof-of-concept to a validation in laboratory environment.Owing to the high surface-area-to-volume ratio, the 8 nm NPSP are more biologically active than the 200 nm NPSP. The 8 nm NPSP thus exhibited a six-fold higher pectinase loading capacity (240 mg g−1) compared to the 40 mg g−1 exhibited by 200 nm NPSP. As a result, there was a significant difference in the rate of fouling defined at various flux (Fig. 12a) and feed concentration (Fig. 12b). At the lower range, they both exhibited similar performance. This could be attributed to the presence of high amounts of immobilized enzyme in both systems that can satisfy the hydrolysis of foulants transported at lower flux or feed concentration. However, with increased flux or feed concentration, while the system with high enzyme loading continued to provide limited rate of fouling, the system with low enzyme loading revealed an almost exponentially rising rate of fouling.
On the other hand, when the 200 nm NPSP was used, an external magnetic field was applied only to assist with the attraction of the NPSP towards the surface of the membrane and easy dispersion on the surface of the membrane. Once the particles were uniformly dispersed on the membrane, the particles were retained based on size exclusion. Hence, it avoided the need for constant application of an external magnetic field throughout the operation. On the contrary, for the 8 nm particles applying the external magnetic field constantly was highly imperative as retention on the membrane is based on magnetic interaction between the NPSP and magneto-responsive membrane. In Fig. 9b, the system efficiency was effectively enhanced by increasing the amount of EnzSP per membrane area without a significant molecular crowding. Hence, one can benefit from the easy retainability and commercial availability of the present NPSP, while boosting the system efficiency to the required level through the possible high enzyme loading.
Yet, both systems exhibited similar trends when operating parameters were varied, i.e. the BMRSP exhibited good reproducibility with a commercial NPSP. Scaling-up and commercialization of the developed BMRSP can thus be smooth since it avoids the complex technical and practical challenges for standardization and mass production of NPSP.
In previous literature, a number of bioinspired engineered nanobeads, such as hybrid silver–silica nano-composites,18 pure silica beads,19 and graphene–bismuth nanocomposites20 have been used to immobilize enzymes in order to prevent leaching and enzyme loss during recycling. Yet, the number of available information regarding the use of these nanobeads as anti-foulants in membrane operation is very limited. Even for those reported in literature, there is very limited evidence on how to easily separate neutral nanobeads from the rest of other retained components, which holds similar particles size. Most of the nanobeads used so far are being separated based on size exclusion. For instance, in the work of Zanoni et al.,21 a 500 nm Si-nanobeads with immobilized enzyme that was used to distract biofilm growth were recovered using 0.45 μm filters. However, due to the presence of residual gels that could not be isolated from the Si-nanobeads using a mere filtration, the recycled Si-nanobeads exhibited reduced mechanical or enzymatic activity on the biofilms. As a result, authors suggested the need to develop an alternative and highly efficient recovery and cleaning methodology.21 In this work, these challenges are resolved by using magneto-responsive nanobeads.
4. Conclusions
The use of bionanocomposites as enzyme carrier to keep membrane surfaces clean, not only protects the membrane from damage and increases its working life, but can also be considered a more environmentally friendly solution because it reduces the use of harsh membrane cleaning agents. The present work focused on the study of enzyme immobilization, specifically pectinase on 200 nm commercial NPSP.The enzyme was immobilized by covalent bond on functionalized NPSP using EGDE. WDS elemental mapping revealed that application of an external magnetic field helped with the formation of a thin layer of uniformly dispersed EnzSP on the surface of the membrane. The EnzSP was effective in reducing fouling during MF of pectin-rich solution. Characterization of the EnzSP containing membrane through SEM micrographs well agreed with the hydrodynamic behavior.
It is clear that BMRSP is a useful tool to resolve the various issues related to the current practice of enzyme immobilization techniques e.g., direct integration in the membrane matrix or use of inert carrier particles. The work demonstrated the feasibility of this innovative system in a laboratory environment and provided knowledge useful for other applications other than the used model system. Since any type of enzyme can be easily immobilized on the NPSP, the system is well-suited for biotransformation that involve immobilized enzymes.
Therefore, considering the high reproducibility of the system with commercial NPSP and the modularity of membrane system, retrofitting the BMRSP in an existing process or scaling-up the new system would be relatively easy. The knowledge gained here using this commercial NPSP will then help to avoid the significant effort and investment required in the processes of standardizing the NPSP mass production while benefiting from better possibilities to select commercially available different particle sized NPSP for target biocatalysis.
Acknowledgements
The authors acknowledge the European Union, FESR, MIUR, MSE for the financial support to the project PON Olio Più – PON01_01545, within the framework PON Ricerca e Competitività 2007–2013. We are also grateful to KU Leuven for support in the frame of IDO 06/008 and OT (11/061), and the Flemish Government for the Methusalem and FWO funding (G.0808.10N) and the Federal Government for an IAP grant.References
- M. Darder, P. Aranda and E. Ruiz-Hitzky, Adv. Mater., 2007, 19, 1309–1319 CrossRef CAS.
- N. Miguel-Sancho, O. Bomatí-Miguel, G. Colom, J. P. Salvador, M. P. Marco and J. Santamaría, Chem. Mater., 2011, 23, 2795–2802 CrossRef CAS.
- N. Sozer and J. L. Kokini, Trends Biotechnol., 2009, 27, 82–89 CrossRef CAS PubMed.
- C. G. C. M. Netto, H. E. Toma and L. H. Andrade, J. Mol. Catal. B: Enzym., 2013, 85–86, 71–92 CrossRef CAS.
- C. W. Lim and I. S. Lee, Nano Today, 2010, 5, 412–434 CrossRef CAS.
- B. Lopez-Ruiz, E. Lopez Cabarcos and J. P. Hervas Perez, Stable biocompatible microgels for enzyme immobilization and its use in biosensors amperometricos, Spain patents, 2,320,078, 18 May 2009.
- J. Qiu and S. Wang, Preparation method of double-enzyme glucose sensor based on graphene, China Patent, 1,030,33549, 17 December 2012.
- J. Hou, G. Dong, B. Xiao, C. Malassigne and V. Chen, J. Mater. Chem. A, 2015, 3, 3332–3342 CAS.
- C. Ji, J. Hou and V. Chen, J. Membr. Sci., 2016, 520, 869–880 CrossRef CAS.
- K. Bélafi-Bakó, M. Eszterle, K. Kiss, N. Nemestóthy and L. Gubicza, J. Food Eng., 2007, 78, 438–442 CrossRef.
- L. Giorno and E. Drioli, Trends Biotechnol., 2000, 18, 339–349 CrossRef CAS PubMed.
- A. Y. Gebreyohannes, M. R. Bilad, T. Verbiest, C. M. Courtin, E. Dornez, L. Giorno, E. Curcio and I. F. J. Vankelecom, J. Membr. Sci., 2015, 487, 209–220 CrossRef CAS.
- A. Y. Gebreyohannes, R. Mazzei, E. Curcio, T. Poerio, E. Drioli and L. Giorno, Ind. Eng. Chem. Res., 2013, 52, 10396–10405 CrossRef CAS.
- E. Bach and E. Schollmeyer, Anal. Biochem., 1992, 203, 335–339 CrossRef CAS PubMed.
- J. V. Diaz, G. E. Anthon and D. M. Barrett, J. Agric. Food Chem., 2007, 55, 5131–5136 CrossRef CAS PubMed.
- Y. Sahoo, H. Pizem, T. Fried, D. Golodnitsky, L. Burstein, C. N. Sukenik and G. Markovich, Langmuir, 2001, 17, 7907–7911 CrossRef CAS.
- F. Militano, T. Poerio, R. Mazzei, E. Piacentini, A. Gugliuzza and L. Giorno, Colloids Surf., B, 2016, 143, 309–317 CrossRef CAS PubMed.
- S. K. Das, M. M. Khan, T. Parandhaman, F. Laffir, A. K. Guha, G. Sekaran and A. B. Mandal, Nanoscale, 2013, 5, 5549–5560 RSC.
- J. Kim, J. W. Grate and P. Wang, Chem. Eng. Sci., 2006, 61, 1017–1026 CrossRef CAS.
- V. Mani, R. Devasenathipathy, S.-M. Chen, B. Subramani and M. Govindasamy, Int. J. Electrochem. Sci., 2015, 10, 691–700 Search PubMed.
- M. Zanoni, O. Habimana, J. Amadio and E. Casey, Biotechnol. Bioeng., 2016, 113, 501–512 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra20455d |
| This journal is © The Royal Society of Chemistry 2016 |