Enhancing the electrochemistry performance of Li4Ti5O12 for Li-ion battery anodes by a sol–gel assisted molten salt method and graphene modification
Qingjun Guo ,
Qiang Wang,
Gang Chen,
Qixin Shen and
Bing Li*
,
Qiang Wang,
Gang Chen,
Qixin Shen and
Bing Li*
East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, P. R. China. E-mail: drlibing@163.com; bingli@ecust.edu.cn
First published on 14th November 2016
Abstract
Graphene modified Li4Ti5O12 composites (G-LTO) were prepared via a sol–gel assisted molten salt synthesis process. The product was fabricated by controlled hydrolysis of tetrabutyl titanate in the presence of graphene oxide, glacial acetic acid and LiCl–KCl molten salts in a mixed solvent of ethanol and water, followed by calcination treatment and a washing process. For comparison, pure LTO and carbon modified LTO were also synthesized by the same process without graphene oxide addition and with urea, respectively. The results reveal that pure LTO has a decreased particle size, and primary particles aggregation and agglomeration is effectively avoided by this method. Besides, the introduction of graphene sheets leads to the most uniform distribution particle size of LTO, and enhances the conductivity between the adjacent LTO particles. Hence, G-LTO composites present an improved rate performance (111.3 mA h g−1 at 10C) and cycling stability (the capacity loss of 11.9% after 500 cycles at 1C). Electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) well explain the electrode kinetics. The charge transfer resistance at the LTO active substance/electrolyte interface is reduced from 316.9 Ω to 151.8 Ω. The Li+ diffusion coefficient in the G-LTO electrode is 7.83 × 10−11 cm2 s−1, which is larger than the 5.49 × 10−11 cm2 s−1 of the P-LTO electrode. The results could be attributed to the smaller particle size, reduced aggregation and improved electronic conductivity as a result of the improved synthetic method and the presence of graphene sheets.
1. Introduction
In recent years, spinel lithium titanate, Li4Ti5O12 (LTO), has been extensively studied as an anode material for lithium ion batteries (LIBs) due to its zero volume change during lithiation de-intercalation and excellent safety owing to its high and stable discharge and charge plateau (about 1.55 V vs. Li/Li+).1–4 Nevertheless, pristine LTO suffers from a relatively poor rate performance due to its inherently low electronic conductivity (∼10−13 S cm−1) and moderate lithium-ion diffusion coefficient (10−9 to 10−13 cm2 s−1).5–9 Many approaches have been explored to address this issue, including ion doping, morphology tailoring and nanotechnology, surface modification and coating with conductive carbon or metal materials and the introduction of some excellent conductive components.10–23Recently, graphene has attracted great attention used in the field of LIBs and other electrochemical devices, due to its extraordinary electronic conductivity (64 mS cm−1), superior thermal properties and mechanical strength, extremely high surface area (2675 m2 g−1).24,25 Hence, preparation LTO/graphene composites or graphene modified LTO are effective ways to improve the electrochemistry performance of LTO and has been widely investigated by variable methods.24–34 For example, Guo, Xiang, et al.24 and Liu, Wen, et al.30 both prepared Li4Ti5O12/graphene composites by a solid state method. Chen, Huang, et al.33 used a small amount of reduce graphene oxide (rGO) nanosheets modified Li4Ti5O12 via a hydrothermal reaction with subsequent annealing. Zhu, Duan, et al.28 created a solution fabrication process with an action of solvothermal treatments to prepare Li4Ti5O12/graphene composite. Xiang, Tian, et al.25 fabricated Li4Ti5O12/graphene composite by sol–gel method. Recently, nanosized Li4Ti5O12/graphene materials have also been synthesized by a sol–gel method, which exhibit an average particle size of approximately 200–500 nm.27 Although Li4Ti5O12/graphene or rGO modified LTO composites mentioned above exhibited good electrochemical performance, it still remains a great challenge to develop a facile, low energy consumption and mass preparation route to achieve good performance Li4Ti5O12/graphene composites.
Here, we proposed a sol–gel assisted molten salt method with a small amount of graphene oxide (GO) addition to gain graphene modified LTO composites. At the pretreatment process, tetrabutyl titanate (TBT) as the Ti source, lithium acetate (LiAc) as the Li source, eutectic LiCl–KCl, as well as dispersive GO as the additive were used in the sol–gel process to get precursor mixture. Then the precursor mixture was heated to 750 °C for 2 h to prepare graphene modified LTO composites in the molten LiCl–KCl melts. As the mixtures of reactants for LTO always stay in a homogenous LiCl–KCl molten salts media. This can not only avoid products agglomeration by general sol–gel method, but also can decrease the particle size than single molten salt method, at the same time, the advantages of molten salts method of homogeneity and high crystallinity are remained. The prepared graphene modified Li4Ti5O12 via the sol–gel assisted molten salt method shows the most uniform distribution and good electrochemical performance. To the best of our knowledge, there was no report to prepare the graphene modified Li4Ti5O12 composites via this method.
2. Experimental
2.1. Materials synthesis
Graphene modified LTO composites were synthesized by a sol–gel assisted molten salt synthesis process. First, GO (0.115 g, 5 wt% of the theoretical LTO, JH-1, 99 wt%, Tianjin CYG) was ultrasound for 1 h in ethyl alcohol (10 mL). Then, the solution was prepared by addition of TBT (8.5 mL), well ultrasonic GO, glacial acetic acid (3.8 mL) into ethyl alcohol (30 mL) at a flask in sequence. LiAc (1.34 g), LiCl (2.54 g) and KCl (3.06 g) were dissolved in deionized water (10 mL) and the above solution were slowly added in drops to the flask under vigorously stirring. A gel was formed after stirring for 20 min. The resulting gel was aged in the air for 3 h and dried at 80 °C for 12 h, then calcined at 750 °C for 2 h under Ar atmosphere. After naturally cooling to room temperature, the mixture was washed with deionized water repeatedly to remove the residual LiCl and KCl. The final powder was obtained after drying at 90 °C for 12 h in a drying oven in air. For comparison, pure LTO and carbon modified LTO powders were prepared in a similar way without GO or with urea (2.004 g, 10 wt% of the theoretical LTO) additive, respectively. The obtained products were named as G-LTO, P-LTO, C-LTO, respectively.In addition, the other pure LTO was also obtained by general sol–gel method.35,36 First, TBT (8.5 mL) was added into ethyl alcohol (30 mL) at a flask, then glacial acetic acid (3.8 mL) and LiAc (1.34 g) dissolved into deionized water (10 mL) solution were gradually dropped into the flask under vigorous stirring. A milk white gel was formed after stirring for 20 min. The resulting gel was aged in the air for 3 h and dried at 80 °C for 12 h, then calcined at 750 °C for 2 h in the air. The final product was obtained and called as PS-LTO.
2.2. Cell assembly
CR 2016 type coin cells assembly were used for electrochemical measurements. A slurry was prepared by mixing the obtained LTO (80 wt%), carbon black (Super-P, 10 wt%), and polyvinylidene fluoride (PVDF, 10 wt%), dissolved in N-methyl-2-pyrrolidine (NMP). All the cells were assembled under the same procedure as our previous report.37 The total electrode loading weight for G-LTO, P-LTO and C-LTO was controlled at about 1.2 mg cm−2.2.3. Material characterization and electrochemical measurements
The structure of the obtained products were characterized by X-ray diffraction measurement (XRD, D/max2550 V, Rigaku Co., Japan) with Cu Kα1 (10° < 2θ < 80°) radiation (λ = 1.54 Å). The thermogravimetric-differential thermal analysis (TG-DTA) was performed on a TG-DT Analyzer (PerkinElmer Pyris Diamond TG/DTA) with a heating rate of 10 °C min−1 from 40 to 700 °C in air. The morphology, energy dispersive spectroscopy and microstructure images were observed with field-emission scanning electron microscopy (FESEM, Merlin Compact), scanning electron microscope (SEM, JSM-6360LV, JEOL, Japan) equipped with Energy Dispersive Spectroscopy (EDS) and transmission electron microscope (TEM, JEM-2100, JEOL, Japan) operated at an accelerating voltage of 200 kV.Galvanostatic charge–discharge measurements were carried out under different current densities (a current density of 175 mA g−1 represents for 1C rate) in the voltage range of 1.0–2.5 V (vs. Li/Li+) using a CT-3008 Battery Test System (New ware Electronics Co. Ltd, Shenzhen). Cyclic voltammetry (CV) was tested by ZJ-100 electrochemical test system (Shanghai Zhengfang Electric Co. Ltd.) with the various scan rates ranging from 0.1 to 5 mV s−1 between 0.5 and 3 V potential. Electrochemical impedance spectroscopy (EIS) were measured by Parstat 2237 electrochemical workstation in the frequency range of 1 Hz to 100 kHz at a signal amplitude of 5 mV. Both CV and EIS were used two-electrode system with LTO electrode as the working electrode and lithium metal as the counter electrode.
3. Results and discussion
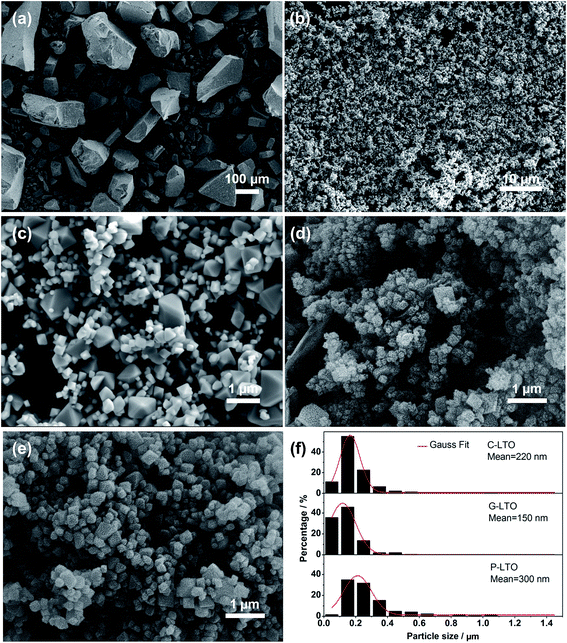
The XRD patterns of P-LTO, G-LTO, C-LTO samples are shown in Fig. 1a. All the samples show the major diffraction peaks, which are in accordance with the spinel Li4Ti5O12 structure with the Fd3m space group (JCPDS card no. 49-0207, the dark blue peaks at the bottom). However, the peaks of all the samples around 27 and 55 degree can be indexed to the anatase TiO2 (JCPDS card no. 21-1276), which possibly results from the volatilize of Li during high-temperature synthesis. This can be avoided through providing excess Li source in the later experiments. No any graphitic peaks are detected in the XRD patterns of G-LTO, C-LTO samples, mainly due to the amorphous structure of graphene and carbon.24,38 In order to confirm the content of graphene in G-LTO sample and carbon in C-LTO sample, TG-DTA was carried out in Fig. 1b and c. In the first stage (40–250 °C), the preliminary weight loss are about 0.79 wt%, 0.53 wt%, respectively, mainly due to the evaporation of the adsorption water on the surface of samples. In the second stage (250–550 °C), the weight loss reach to 3.34 wt%, 1.51 wt%, which are the content of graphene in G-LTO sample and carbon in C-LTO sample, respectively. So the results indicate that small amount of graphene or carbon addition have no impact on the crystal structure and crystallinity of spinel Li4Ti5O12 in this method.Fig. 2a–e shows FESEM images of PS-LTO sample, P-LTO sample at a low and high magnification, and SEM images of G-LTO, C-LTO samples, respectively. As shown in Fig. 2a, the FESEM image of PS-LTO sample via general sol–gel method shows severe agglomeration to bulk LTO. However, the FESEM image of P-LTO sample in Fig. 2b through sol–gel assisted molten salt method reveals favourable dispersity without agglomeration. FESEM image of P-LTO, SEM images of G-LTO and C-LTO samples in Fig. 2c–e reveal that all the products are well-crystallized and composed of octahedron particles. And in Fig. 2d, a small amount of graphene sheets are found between the G-LTO particles.
The histograms in Fig. 2f show the particle size distribution and average particle size of P-LTO, G-LTO and C-LTO samples calculated from the SEM images (in Fig. 2c–e) by Nano Measure software. G-LTO sample shows relatively homogenous particle size ranging from 10–530 nm. P-LTO and C-LTO samples are mainly in a range of 60–1080 nm and 40–1020 nm, respectively. The average particle sizes are 300 nm for P-LTO, 150 nm for G-LTO, 220 nm for C-LTO, respectively. GO and urea modified LTO samples present more narrower particle size distribution than P-LTO. Especially, nano-particles occupy a large proportion in G-LTO sample. So the introduction of graphene or carbon in this method is not only helpful to make uniform particle size, but also suppress the growth of Li4Ti5O12 particles.
Energy Dispersive Spectroscopy (EDS) and mapping results in Fig. 3 reveal the weight ratio of C element in G-LTO and C-LTO are 2.37 wt% and 3.06 wt%, respectively. And the distribution of C elemental is completely corresponding to that of LTO particles, which further confirm the existence the formation of C element in the resulting samples. Combined with the results of SEM images, we can conclude that the graphene and carbon mainly distribute on the surface of G-LTO and C-LTO particles, which can be confirmed by the following TEM test.
 | ||
| Fig. 3 SEM images of (a) G-LTO and (b) C-LTO samples with the corresponding EDS and mapping images of C, O, and Ti elements. | ||
TEM images in Fig. 4 show the graphene sheets or carbon layer are on the surface and between G-LTO or C-LTO particles, respectively. In the inserted image of Fig. 4b and in Fig. 4d, the high resolution TEM images of G-LTO and C-LTO samples give a well-crystallized structure with 0.48 nm lattice spacing, matches well with the spacing of (111) plane of Li4Ti5O12. These provide a further evidence for the graphene sheets or carbon layer contact with LTO particles, giving a connection between adjacent LTO particles, as well as preventing them from further growth. Graphene sheets or carbon layer can enhance electronic conductivity of LTO. Smaller particle size also means shorter lithium ions diffusion distance. So the introduced graphene or carbon is supposed to enhance the electrochemistry performance of LTO.
 | ||
| Fig. 4 TEM images of (a and b) G-LTO and (c) C-LTO samples. HRTEM images of (inserted in b) G-LTO and (d) C-LTO samples. | ||
Fig. 5a compares the rate performance among P-LTO, G-LTO and C-LTO electrodes. It is clear that the G-LTO electrode exhibits an improved rate performance among the three electrodes. At the rate of 10C (charge/discharge in 6 min, 1.75 A g −1), a specific discharge capacity of 111.3 mA h g−1 can be retained for G-LTO electrode, which is much better than that of P-LTO electrode (68.5 mA h g−1) and C-LTO electrode (71.4 mA h g−1). Fig. 5b shows and the first discharge/charge curves of P-LTO, G-LTO and C-LTO electrodes at various rates from 0.15C to 10C. All the electrodes show flat voltage plateaus around 1.55 V (vs. Li/Li+) at different current rates, which ensure a constant potential output for LIBs. Combined to the rate performance in Fig. 5a, the G-LTO electrode can deliver the discharge capacities of 172.9, 148.4, 142.5, 140.3, 137.4, 133.1 and 122.7 mA h g −1 in the rate of 0.15C, 0.2C, 0.5C, 0.75C, 1C, 2C and 5C, respectively. For P-LTO electrode, the corresponding discharge capacities only are 165.7, 142.5, 133.7, 122.8, 115.0, 105.7 and 82.6 mA h g−1, respectively.
The cycling performance and coulombic efficiency of the P-LTO, G-LTO and C-LTO electrodes at 0.2C rate in the first 5 cycles, and the following 500 cycles at 1C rate are shown in Fig. 5c–e, respectively. For P-LTO electrode, the initial discharge capacity is 165.7 mA h g−1, after 5 cycles and the current rate raising from 0.2C to 1C, it decreases to 137.7 mA h g−1 with a capacity retention of 83.1%, delivers the capacity of only 121.3 mA h g−1 after the following 500 cycles at 1C rate (capacity loss of 11.9%). In the case of the G-LTO electrode, it delivers the initial discharge capacity of 172.8 mA h g−1 with the first coulombic efficiency of 85.9%, then 146.9 mA h g−1 with a capacity retention of 85% after raising to 1C, and the capacity of 129.4 mA h g−1 (capacity loss of 11.9%) after the following 500 cycles at 1C rate, demonstrating an improved cycling performance. In Fig. 5e, C-LTO electrode receives the first discharge capacity of 170.9 mA h g−1 and coulombic efficiency of 81.5%, then 134.5 mA h g−1 with a capacity retention of 78.7% after raising to 1C, and capacity loss of 11.2% after the following 500 cycles at 1C rate. From the second cycle onward, the three electrodes all receive the high recharge coulombic efficiency close to 100%. Fig. 5f shows the 6th, 50th, 100th, 200th, 300th, 500th discharge/charge curves of G-LTO electrodes, all the curves show a flat potential plateau around 1.55 V (vs. Li/Li+), corresponding with the reduction/oxidation reaction between Li4Ti5O12 and Li7Ti5O12 during Li+ intercalation/deintercalation processes, which means there is no other reduction/oxidation reaction in the discharge/charge processes. The results can be verified by the cyclic voltammetry (CV) results.
To gain more information on the electrode kinetics, the EIS test and the simulation equivalent circuit of P-LTO, G-LTO and C-LTO electrodes are shown in Fig. 6. Generally, the intercept at the Z′ axis in the high frequency region corresponds to the ohmic resistance (Rs) of electrode and electrolyte, and the semicircle in the high-to-mid frequency region is attributed to the Li+ charge-transfer resistance (Rct) on the electrode–electrolyte interface. The sloping line in the low frequency region represents Li+ diffuse resistance, also called Warburg impedance (W).39,40 According to the equivalent circuit inserted in Fig. 6, the Rs and Rct of G-LTO electrode are 2.57 Ω and 151.8 Ω, respectively, which are much lower than that of P-LTO electrode (3.51 Ω and 316.9 Ω). For C-LTO electrode, the value of Rs and Rct are 3.85 Ω and 183.6 Ω, respectively. The largest slope of G-LTO electrode indicates the highest Li+ mobility among the three electrodes. These confirm that graphene modification in LTO here improves the electronic conductivity and lithium ion diffusion of G-LTO composites, which could contribute to the enhanced rate capability.
 | ||
| Fig. 6 Electrochemical impedance spectra (EIS) of P-LTO, G-LTO and C-LTO electrodes with insert of the simulation equivalent circuit and partial enlarged view for the EIS. | ||
To verify the effects of the introduction of graphene on the electrochemical polarization and the Li+ diffusion coefficient of the G-LTO electrode, cyclic voltammetry (CV) of P-LTO, G-LTO and C-LTO electrodes are obtained at various scan rates, as shown in Fig. 7. All the curves show a pair of peaks, corresponding to the reduction/oxidation reaction between Li4Ti5O12 and Li7Ti5O12 during Li+ intercalation/deintercalation process. At the scan rate of 0.1 mV s−1, the polarization between the reduction and oxidation peaks is 0.19 V for G-LTO (Fig. 7a), which is much lower than that of P-LTO (0.27 V, Fig. 7b) and C-LTO (0.22 V, Fig. 7c). Then with the increasing of scan rate, the reduction peaks shift to more negative potentials and the oxidation peaks shift to more positive one. The G-LTO and C-LTO electrodes exhibit smaller potential shift between the reduction and oxidation peaks than P-LTO electrode, indicating that the G-LTO and C-LTO electrodes have lower polarization due to fastest electrode kinetics by graphene or carbon modification. Moreover, the reduction peak and oxidation peak currents for G-LTO electrode are larger than that of P-LTO and C-LTO electrodes, implying that the electrochemical reaction rate is enhanced by adding graphene due to a smaller particle size and increased electronic conductivity of LTO.
 | ||
| Fig. 7 CV plots of (a) P-LTO, (b) G-LTO and (c) C-LTO electrodes at various scan rates ranging from 0.1 to 5.0 mV s−1. Insets: the reduction peak current (ip) vs. the square root of scan rate (v1/2). | ||
The relationship between the reduction peak current (ip) and the square root of the scan rate (v1/2, V s−1) for the P-LTO and G-LTO electrodes in the inserted images in Fig. 7a and b have shown a linearity passing through the origin, implying that the reduction/oxidation process in the electrode material (solid-state LTO) is controlled by Li+ diffusion.41–43 The diffusion coefficient (D, cm2 s−1) of Li+ in the electrode could be calculated by the Randles–Sevcik equation in eqn (1).41,44
| ip = 0.4463ACLi+(nF)3/2(Dv/RT)1/2 | (1) |
4. Conclusions
The homogeneous graphene modified LTO composites with highly crystallinity and favourable dispersity has been prepared by a sol–gel assist molten salt method at a lower reaction temperature and shorter holding time. This method is not only helpful to decrease the particle size, but also effective to restrict aggregation and agglomeration of the LTO particles. The introduction of graphene sheets in the G-LTO leads to the most uniform distribution of LTO, and improves the conductivity between the adjacent LTO particles, therefore, enhances the electrochemistry performance of LTO. The EIS and CV measurements show that the G-LTO composites possess a lower impedance, higher lithium ion diffusion coefficient than P-LTO.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 51474107).References
- S. Chen, Y. Xin, Y. Zhou, Y. Ma, H. Zhou and L. Qi, Energy Environ. Sci., 2014, 7, 1924 CAS.
- W. Chen, H. Jiang, Y. Hu, Y. Dai and C. Li, Chem. Commun., 2014, 50, 8856 RSC.
- T. Yuan, W.-T. Li, W. Zhang, Y.-S. He, C. Zhang, X.-Z. Liao and Z.-F. Ma, Ind. Eng. Chem. Res., 2014, 53, 10849 CrossRef CAS.
- X. Sun, P. V. Radovanovic and B. Cui, New J. Chem., 2015, 39, 38 RSC.
- F. Zhang, B. Xu, G. Cao, M. Chu, N. Qiao, G. Wei and Y. Yang, RSC Adv., 2014, 4, 53981 RSC.
- H.-K. Kim, K. C. Roh, K. Kang and K.-B. Kim, RSC Adv., 2013, 3, 14267 RSC.
- X. Lu, L. Gu, Y. S. Hu, H. C. Chiu, H. Li, G. P. Demopoulos and L. Chen, J. Am. Chem. Soc., 2015, 137, 1581 CrossRef CAS PubMed.
- L. Zhao, Y. S. Hu, H. Li, Z. Wang and L. Chen, Adv. Mater., 2011, 23, 1385 CrossRef CAS PubMed.
- J. Liu, K. Song, P. A. Aken, J. Maier and Y. Yu, Nano Lett., 2014, 14, 2597 CrossRef CAS PubMed.
- L. Cheng, J. Yan, G.-N. Zhu, J.-Y. Luo, C.-X. Wang and Y.-Y. Xia, J. Mater. Chem., 2010, 20, 595 RSC.
- W. Li, H. Wang, M. Chen, J. Gao, X. Li, W. Ge, M. Qu, A. Wei, L. Zhang and Z. Liu, Electrochim. Acta, 2016, 188, 499 CrossRef CAS.
- Q. Zhang, Y. Liu, H. Lu, D. Tang, C. Ouyang and L. Zhang, Electrochim. Acta, 2016, 189, 147 CrossRef CAS.
- L. Peng, H. Zhang, L. Fang, Y. Zhang and Y. Wang, Nanoscale, 2016, 8, 2030 RSC.
- C.-C. Yang, H.-J. Hwu, S. J. Lin, W.-C. Chien and J.-Y. Shih, Electrochim. Acta, 2014, 125, 637 CrossRef CAS.
- W. Yao, W. Zhuang, X. Ji, J. Chen, X. Lu and C. Wang, Mater. Res. Bull., 2016, 75, 204 CrossRef CAS.
- C. Wang, S. Wang, L. Tang, Y.-B. He, L. Gan, J. Li, H. Du, B. Li, Z. Lin and F. Kang, Nano Energy, 2016, 21, 133 CrossRef CAS.
- Y. Tang, L. Liu, H. Zhao, D. Jia and W. Liu, J. Mater. Chem. A, 2016, 4, 2089 CAS.
- L. Sun, W. Kong, H. Wu, Y. Wu, D. Wang, F. Zhao, K. Jiang, Q. Li, J. Wang and S. Fan, Nanoscale, 2016, 8, 617 RSC.
- C. P. Sandhya, B. John and C. Gouri, J. Alloy. Compd., 2016, 655, 238 CrossRef CAS.
- X. Jia, Y. Lu and F. Wei, Nano Res., 2016, 9, 230 CrossRef CAS.
- B. Zhao, R. Ran, M. L. Liu and Z. P. Shao, Mat. Sci. Eng., R, 2015, 98, 1 CrossRef.
- T.-F. Yi, S.-Y. Yang and Y. Xie, J. Mater. Chem. A, 2015, 3, 5750 CAS.
- J. Liu, K. Tang, K. Song, P. A. Aken, Y. Yu and J. Maier, Phys. Chem. Chem. Phys., 2013, 15, 20813 RSC.
- X. Guo, H. F. Xiang, T. P. Zhou, W. H. Li, X. W. Wang, J. X. Zhou and Y. Yu, Electrochim. Acta, 2013, 109, 33 CrossRef CAS.
- H. Xiang, B. Tian, P. Lian, Z. Li and H. Wang, J. Alloys Compd., 2011, 509, 7205 CrossRef CAS.
- Y. Tang, F. Huang, W. Zhao, Z. Liu and D. Wan, J. Mater. Chem., 2012, 22, 11257 RSC.
- H.-p. Liu, G.-w. Wen, S.-f. Bi, C.-y. Wang, J.-m. Hao and P. Gao, Electrochim. Acta, 2016, 192, 38 CrossRef CAS.
- J. Zhu, R. Duan, Y. Zhang and J. Zhu, Ceram. Int., 2016, 42, 334 CrossRef CAS.
- S. G. Ri, L. Zhan, Y. Wang, L. Zhou, J. Hu and H. Liu, Electrochim. Acta, 2013, 109, 389 CrossRef CAS.
- H. Liu, G. Wen, S. Bi and P. Gao, Electrochim. Acta, 2015, 171, 114 CrossRef CAS.
- D. Kong, W. Ren, Y. Luo, Y. Yang and C. Cheng, J. Mater. Chem. A, 2014, 2, 20221 CAS.
- W.-T. Li, T. Yuan, W. Zhang, J. Ma, C. Zhang, Y.-S. He, X.-Z. Liao and Z.-F. Ma, J. Power Sources, 2015, 285, 51 CrossRef CAS.
- C. Chen, Y. Huang, H. Zhang, X. Wang, G. Li, Y. Wang, L. Jiao and H. Yuan, J. Power Sources, 2015, 278, 693 CrossRef CAS.
- Y. Oh, S. Nam, S. Wi, J. Kang, T. Hwang, S. Lee, H. H. Park, J. Cabana, C. Kim and B. Park, J. Mater. Chem. A, 2014, 2, 2023 CAS.
- Y.-C. Kuo and J.-Y. Lin, Electrochim. Acta, 2014, 142, 43 CrossRef CAS.
- C. Shen, X. Zhang, Y. Zhou and H. Li, Mater. Chem. Phys., 2002, 78, 437–441 CrossRef.
- Q. Guo, S. Li, H. Wang, Y. Gao and B. Li, RSC Adv., 2014, 4, 60327 RSC.
- L. Shen, C. Yuan, H. Luo, X. Zhang, S. Yang and X. Lu, Nanoscale, 2011, 3, 572 RSC.
- T.-F. Yi, J.-Z. Wu, M. Li, Y.-R. Zhu, Y. Xie and R.-S. Zhu, RSC Adv., 2015, 5, 37367 RSC.
- D. Mu, Y. Chen, B. Wu, R. Huang, Y. Jiang, L. Li and F. Wu, J. Alloys Compd., 2016, 671, 157 CrossRef CAS.
- Y. Gao, Y. Shi, X. Liu, C. Huang and B. Li, Electrochim. Acta, 2016, 190, 208 CrossRef CAS.
- C. Huang, X. Liu, Y. Gao, S. Liu and B. Li, Faraday Discuss., 2016, 190, 339 RSC.
- S. Liu, L. Chen, B. Li, L. Wang, B. Yan and M. Liu, Electrochim. Acta, 2014, 147, 82 CrossRef CAS.
- Y. Yang, B. Qiao, X. Yang, L. Fang, C. Pan, W. Song, H. Hou and X. Ji, Adv. Funct. Mater., 2014, 24, 4349 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |