Synthesis of core–shell imprinting polymers with uniform thin imprinting layer via iniferter-induced radical polymerization for the selective recognition of thymopentin in aqueous solution
Xumian Gao *,
Xiaoling Hu*,
Ping Guan,
Chunbao Du
*,
Xiaoling Hu*,
Ping Guan,
Chunbao Du ,
Shichao Ding,
Xiaoyan Zhang,
Bangpeng Li,
Xiongqi Wei and
Renyuan Song
,
Shichao Ding,
Xiaoyan Zhang,
Bangpeng Li,
Xiongqi Wei and
Renyuan Song
Department of Applied Chemistry, Key Laboratory of Applied Physics and Chemistry in Space of Ministry of Education, School of Natural and Applied Science, Northwestern Polytechnical University, Xi'an 710072, P. R. China. E-mail: gaoxumian@163.com; huxl@nwpu.edu.cn; Fax: +86-29-88431639; Tel: +86-29-88431639
First published on 4th November 2016
Abstract
An approach for synthesizing core–shell imprinting polymers using P(EGDMA-CMS) microspheres prepared via dispersion polymerization as a core and employing a surface imprinting technique and iniferter-induced radical polymerization is described. N,N-Diethyldithiocarbamyl groups were immobilized on the surface of the supporting microspheres to form the surface iniferter and further prepare the imprinting layer. Thymopentin (TP5) was selected as the template molecule, which was known to be an immunomodulating agent that had medical properties. Here, a bifunctional ionic liquid (IL), namely, 1-vinyl-3-carbamoylmethyl-imidazolium chloride ([VACMIM]Cl), was synthesized and employed as a novel functional monomer on the basis of the demands of peptide imprinting and the designability of ILs. Under irradiation by UV light, the surface iniferter decomposed and then polymerization was initiated to form a thin surface imprinting layer with specific recognition cavities for TP5. The surface imprinting layer possessed a uniform thickness of ∼35 nm, which was beneficial for the mass transfer of the template TP5, owing to good control of the thickness of the imprinting layer by controlled/living radical polymerization (CRP). The polymeric microspheres were fully characterized and their adsorption properties were investigated. The surface molecular imprinting microspheres (SMIMs) displayed high binding affinity, good selective specificity, rapid adsorption equilibrium and satisfactory reusability. The Scatchard plots of the SMIMs could be fitted to one straight line, which suggested that there was only one kind of binding site. Furthermore, the method of combining a surface imprinting technique and CRP together can be extended to a wide range of applications for chemical sensors, drug delivery and the separation of biomacromolecules.
1. Introduction
Thymopentin (TP5, Fig. 1) is a synthetic water-soluble pentapeptide (amino acid sequence: Arg-Lys-Asp-Val-Tyr), which corresponds to the active site of thymopoietin.1,3 As an immunomodulating agent, TP5 is clinically used in the treatment of autoimmune diseases such as atopic dermatitis, chronic lymphocytic leukemia, Sezary's syndrome, and rheumatoid arthritis, as well as decreased immunocompetence in older surgical patients.2–5 Besides, TP5 is also found to be beneficial in the treatment of some serious illnesses including cancer and AIDS.6,7 Furthermore, researchers have found that TP5 could reduce “typical” inflammatory responses, which may be promising as a disease prevention mechanism, thereby diminishing the risk of fatal consequences of multiple sclerosis in humans.8 So far, TP5 has usually been separated and purified by two methods, namely, ion-exchange chromatography and reversed-phase chromatography.9,10 However, these two methods have high requirements for instrumentation and finance.9 Therefore, other rapid, efficient and cheap separation methods for accurately recognizing and separating TP5 should be established. In view of the urgent demands, the design and preparation of functional materials with selective and high affinity for TP5 are greatly needed. | ||
| Fig. 1 Chemical structures of the template molecule TP5 (a, pI 8.59) and its analogue IPH (b, pI 3.30) used in this study. | ||
Molecular imprinting technology (MIT) is a technique in which a tailor-made polymer is synthesized for the specific recognition of a template molecule under the guidance of a template molecule.11 It has undergone explosive growth in the past two decades and is currently highly topical.12 It has attracted a great deal of interest owing to its advantages of desirable selectivity, physical robustness, and thermal stability, as well as low cost and easy preparation.13 Typical procedures for synthesizing molecular imprinting polymers (MIPs) include four steps, which are the self-assembly of template molecules and functional monomers, the copolymerization of functional monomers and a cross-linker in a solvent, the removal of template molecules and the rebinding of template molecules in a solution of a certain concentration, respectively.14 MIT has a wide range of applications in various fields including analytical chemistry,15 separation and purification,16–18 electrochemical sensors,19 biosensors,20 drug delivery systems21 and catalysis.22–24 MIPs can be prepared in a variety of physical forms by using different polymerization techniques. However, MIPs prepared by traditional polymerization methods such as bulk polymerization and suspension polymerization have their own shortcomings. For example, MIPs prepared by these traditional methods are irregular in size and shape and can only be efficient on a laboratory scale.25 In addition, a series of problems such as poor accessibility, a low mass transfer rate, nonuniform and deeply embedded imprinting binding sites and difficulties in removing template molecules have also occurred.26 Free-radical polymerization (FRP) techniques are widely used to synthesize polymers for both commercial and lab-scale use. However, a free-radical process does not allow control of the size, architecture, and number of synthesized macromolecules because of chain transfer and termination reactions.27 As a result, FRP is far from ideal for applications in the area of imprinting.
Core–shell (CS) imprinting polymers have frequently been synthesized via different methods, whereas controlled/living radical polymerization methods (CRPs) are an ideal choice owing to their intrinsic advantages. For example, CRPs have great potential in providing controlled morphology and controlled thickness of the grafted layer.28 In addition, CRPs have been introduced into the area of imprinting for the purpose of solving general problems associated with MIPs, in particular, their heterogeneity in terms of their internal morphology and distribution of binding site affinities.29,30 Core–shell imprinting polymers were synthesized with the aid of a surface imprinting technique. Surface imprinting polymers possess the features of a high concentration of binding sites on the surface and low mass transfer resistance. In comparison to traditional MIPs (TR-MIPs), surface imprinting polymers possess not only higher binding capacities but also faster mass transfer and binding kinetics, owing to the nearly complete removal of the template.31 Typical CRPs include iniferter-induced radical polymerization, nitroxide-mediated “living” radical polymerization (NMP), atom transfer radical polymerization (ATRP) and reversible addition–fragmentation chain transfer polymerization (RAFT). CRPs have the ability to improve the morphology of imprinting polymers by controlling polymer chain growth. Among these, iniferter methods are among the most versatile because of their applicability under mild conditions and their compatibility with a wide range of monomers, as compared, for instance, to ATRP, in which the metal catalyst is inhibited by acidic monomers.32 As a result, iniferter polymerization is used to obtain water-compatible polymer microspheres with the aid of a hydrophilic ionic liquid for aqueous-phase imprinting.
Iniferter-induced radical polymerization (Fig. 2) was first proposed by Otsu et al.33 in 1982. Under UV irradiation, the iniferter decomposes into an active radical and an inactive radical, where the active radical is bound to the surface of the supporting microsphere and initiates polymerization, and the inactive radical in solution mainly reacts via chain transfer and terminates the reaction to form a “dormant species”.34 In comparison with traditional radical polymerization, this polymerization process can be well controlled by the iniferter owing to the avoidance of adverse reactions such as radical coupling and disproportionation reactions.35 Mayes36 et al. synthesized molecular imprinting core–shell nanoparticles based on dithiocarbamate iniferters via surface-initiated living-radical polymerization and the molecular imprinting CS nanoparticles successfully recognized the template. Besides, Li37 et al. synthesized a surface-modified molecular imprinting membrane via an iniferter method for the permselective separation of lysozyme. The advantages of employing surface imprinting via iniferter-induced radical polymerization in the area of imprinting can be summarized as follows.30,38 Firstly, evenly distributed and controllable thin layers on MIPs with reduced mass transfer resistance can be created. Secondly, block polymers with different properties can be grafted in the presence of an iniferter. Thirdly, UV irradiation is better than thermal initiation because template/monomer complexes are stable at low temperatures. Fourthly, the system can be re-initiated with the same or other monomers, thus enabling the tailoring of multiple shells with defined layer thicknesses.
 | ||
| Fig. 2 Mechanism of iniferter-induced radical polymerization.14 | ||
Ionic liquids (ILs), which are composed of bulky and asymmetrical organic cations and evenly shaped inorganic or organic anions, are synthetic salts.39 Owing to their many excellent properties such as a nearly negligible vapor pressure, good thermal stability, excellent solubility of organic and inorganic compounds, high density of ions, high ionic conductivity, designability, and so on,40–42 ILs have become promising materials and are employed in many fields including analytical chemistry,43 electrochemistry44,45 and separation processes.46 The power of IL-based materials rests on their dual nature, and their separation mechanisms involve multiple interactions including ion exchange, electrostatic interactions, hydrophobic interactions, and π–π stacking interactions.47,48 Recently, many groups have investigated the roles that ILs can play in the field of molecular imprinting. They have found that ILs can be used as functional monomers,49 templates,50 cross-linkers51 or porogens52 in the polymerization procedure and could improve the selectivity and adsorption capacity of MIPs.53 In general, the functional monomers commonly used in MIT are methacrylic acid (MAA), acrylamide (AM), 4-vinylpyridine (4-VP), N-isopropylacrylamide (NIPAm), and so on. Therefore, the choice of functional monomers is usually limited when the imprinting process occurs in the aqueous phase. In addition, the single interaction taking place between the template and the functional monomer could not guarantee specific recognition and high adsorption capacity simultaneously. The immunomodulatory peptide TP5 was chosen as the template because it contains five amino acid residues, of which two are positively charged, one is negatively charged, one is uncharged and hydrophobic and one is uncharged but polar (with an –OH group). TP5 was fully investigated here in order to synthesize an adequate IL functional monomer. For this purpose, multiple interactions between the functional monomer and TP5 should be designed that would play a predominant role in the adsorption process. Considering that the interactions between TP5 and monomers are vital for promoting the adsorption properties of MIPs, some general concepts should be taken into account.54 Firstly, more selective binding sites are produced via interactions with specific directionality. Secondly, favorable binding and selectivity would arise when multiple interactions between the template and MIPs are present. Thirdly, in bi/multifunctional imprinting, selectivity via interactions with multiple functional groups is better when the intramolecular separation of the groups is maximized. On the basis of the aforementioned principles and the designability of ILs, a polymeric ionic liquid with a terminal amino group would be very suitable to act as the functional monomer of TP5 for the preparation of MIPs.
In this work, polymer microspheres were synthesized via dispersion polymerization and were used as supporting materials in the preparation of surface imprinting microspheres. The surface of the supporting microspheres was first modified with diethyldithiocarbamate trihydrate by a coupling reaction to immobilize iniferter groups. Thereafter, a novel ionic liquid, namely, 1-vinyl-3-carbamoylmethyl-imidazolium chloride ([VACMIM]Cl), which could be used as a functional monomer owing to its C–C double bond and functional group, was synthesized by a one-step alkylation reaction. Then, surface molecular imprinting microspheres (SMIMs) were prepared using DDTC-modified (here, sodium diethyldithiocarbamate trihydrate is denoted as DDTC) microspheres as an active matrix, [VACMIM]Cl as a functional monomer and N,N′-methylenebisacrylamide (MBA) as a cross-linker in the aqueous phase by the “grafting from” approach under ultraviolet light at room temperature. UV irradiation was selected to enable the polymerization of SMIMs in moderate conditions and avoid the poor interactions between the template and the functional groups at high temperatures. The obtained polymers were fully characterized by X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to confirm the success of every step. Moreover, the characterization, adsorption properties, Scatchard analysis, selectivity and reusability of the SMIMs were investigated systematically.
2. Experimental
2.1 Materials
Polyethylene glycol dimethacrylate (PEGDMA, Mn: 340, n ≈ 4, TCI), sodium diethyldithiocarbamate trihydrate (DDTC, Mn: 225.31, Aladdin), 2-chloroacetamide (Aladdin) and phenol (Tianjin Tianli Chemical Reagent Co. Ltd) were used as received. Ethylene glycol dimethacrylate (EGDMA) and N,N′-methylenebisacrylamide (MBA) were supplied by Aladdin and used as cross-linkers. Tris(hydroxymethyl)aminomethane (Tris, Aladdin) and concentrated hydrochloric acid (37.5%) were used to prepare a Tris–HCl buffer solution. Polyvinylpyrrolidone (PVP–K90, Mr: 360![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, K: 88–96, Sigma-Aldrich) was used as a stabilizer. 2,2′-Azobisisobutyronitrile (AIBN, Sigma-Aldrich) was recrystallized from methanol and used as an initiator. Absolute ethyl alcohol and ethyl acetate were supplied by Tianjin Tianli Chemical Reagent Co. Ltd and used as solvents. The monomer 4-(chloromethyl)-styrene (p-CMS, TCI) and 1-vinylimidazole (VI, Shanghai Dibo Chemical Technology Co. Ltd) were used without further purification. TP5 (Mw: 679.8, pI 8.59, Aladdin) was chosen as the template molecule and was used in all the adsorption and desorption studies. Immunostimulating peptide, human (IPH, Mw: 716.8, pI 3.30, Shanghai Apeptide Co. Ltd) is an analogue of TP5 and was used for comparison in the study.
000, K: 88–96, Sigma-Aldrich) was used as a stabilizer. 2,2′-Azobisisobutyronitrile (AIBN, Sigma-Aldrich) was recrystallized from methanol and used as an initiator. Absolute ethyl alcohol and ethyl acetate were supplied by Tianjin Tianli Chemical Reagent Co. Ltd and used as solvents. The monomer 4-(chloromethyl)-styrene (p-CMS, TCI) and 1-vinylimidazole (VI, Shanghai Dibo Chemical Technology Co. Ltd) were used without further purification. TP5 (Mw: 679.8, pI 8.59, Aladdin) was chosen as the template molecule and was used in all the adsorption and desorption studies. Immunostimulating peptide, human (IPH, Mw: 716.8, pI 3.30, Shanghai Apeptide Co. Ltd) is an analogue of TP5 and was used for comparison in the study.
2.2 Apparatus
1H NMR analysis was carried out using a Bruker Advance II 300 MHz spectrometer with DMSO-d6 as the solvent and tetramethylsilane as an internal standard. FT-IR spectra in the region of 4000–400 cm−1 were recorded by the KBr pellet method with a Tensor 27 FTIR spectrometer (Bruker). Characterization of the chemical composition of the surfaces was carried out by XPS (Kratos) and the C 1s, O 1s, N 1s, S 2p, and Cl 2p spectra were recorded after light sputtering with argon ions. All binding energy values were referenced to the C 1s hydrocarbon peak at 284.6 eV. The concentration of TP5 was detected with a UV-2550 (Shimadzu) spectrophotometer at a wavelength of 276 nm. SEM measurements were carried out with a FEI Quanta 400 FEG. TEM (JEOL JEM-3010) was used to investigate the shapes and morphologies of the polymer microspheres, and was an especially efficient tool for the observation and identification of core–shell structures of SMIMs.2.3 Synthesis of 1-vinyl-3-carbamoylmethyl-imidazolium chloride ionic liquid
1-Vinyl-3-carbamoylmethyl-imidazolium chloride (Fig. 3a, [VACMIM]Cl) was synthesized via a one-step alkylation reaction. The experimental procedure was as follows. In a 100 mL flask, 3.0 g 2-chloroacetamide was dissolved in 50 mL ethyl acetate by stirring at 50 °C for about 20 min to obtain a clarified solution. Then, 30 mg phenol was added to the mixture as an inhibitor. Afterwards, 3.0 mL 1-vinylimidazole was added dropwise and then the reaction was carried out for 24 h at 45 °C. The white precipitate that was obtained was washed with ethyl acetate and filtered several times. The product was dried at 30 °C for 3 h in a vacuum oven. | ||
| Fig. 3 Chemical structures of the ionic liquid functional monomer [VACMIM]Cl (a) and the cross-linker MBA (b) used in this study. | ||
1H NMR (DMSO-d6, δ) 9.62 (s, 1H, NC*HN), 8.26 (s, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHNC*H
CHNC*H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.89 (s, 1H, CH
CH), 7.89 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C*HNCH2), 8.12 (s, 1H, N*H2), 7.58 (s, 1H, N*H2), 7.42 (dd, 1H, CH2
C*HNCH2), 8.12 (s, 1H, N*H2), 7.58 (s, 1H, N*H2), 7.42 (dd, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C*HN), 6.02 (d, 1H, C*H2
C*HN), 6.02 (d, 1H, C*H2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.44 (d, 1H, C*H2
CH), 5.44 (d, 1H, C*H2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.1 (s, 2H, NC*H2C).
CH), 5.1 (s, 2H, NC*H2C).
2.4 Synthesis of P(EGDMA-CMS) microspheres
P(EGDMA-CMS) microspheres were synthesized according to the following method. In a typical procedure, PVP-K90 (0.75 g) was dissolved in absolute ethyl alcohol (80 mL) in a 250 mL flask, and then PEGDMA (0.8 mL), p-CMS (1.0 mL) and EGDMA (2.0 mL) were added successively with stirring. Thereafter, the solution was purged with nitrogen for 30 min to remove oxygen. Afterwards, AIBN (76 mg, 2%) was added and the temperature was increased to 70 °C and then kept at 70 °C for 7 h with stirring. After the end of the reaction and after cooling to room temperature, the P(EGDMA-CMS) microspheres that were obtained were washed with ethanol and the washing solution was removed by centrifugation at 5000 rpm for 3 min at each step. Then, the P(EGDMA-CMS) microspheres were dried overnight under vacuum at 30 °C.2.5 Iniferter reagent immobilization on P(EGDMA-CMS) microspheres
In order to form the surface iniferter, DDTC was immobilized on the surface of P(EGDMA-CMS) microspheres by a coupling reaction using ethanol as a solvent. The product (denoted as P(EGDMA-CMS)@DDTC) was obtained as follows. At first, in a 100 mL three-necked round-bottom flask, which was equipped with a reflux condenser, a magnetic stirrer and a dropping funnel, were placed 0.5 g P(EGDMA-CMS) microspheres that had been dispersed in 30 mL ethanol by ultrasonic vibration for 10 minutes. Then, a solution of DDTC (5.0 g, 22.2 mmol) in 30 mL ethanol was added dropwise at 0 °C in dark conditions with gentle stirring for 2 h. The reaction was then continued with stirring for 24 h at room temperature. Sodium chloride crystals were precipitated during the reaction. Finally, the products were washed with ethanol and centrifuged several times until the supernatant was clarified. After centrifugation, the DDTC-modified microspheres were dried at 30 °C under vacuum overnight in the dark and stored in the dark until being used.2.6 Preparation of surface imprinting layer
The surface imprinting layer was synthesized via iniferter-induced radical polymerization under ultraviolet irradiation. As shown in Scheme 1, a pre-polymer solution was prepared by dissolving [VACMIM]Cl (0.5 mmol), MBA (2.0 mmol) and the template TP5 (10 μmol) in 10 mL Tris–HCl buffer solution (0.05 mol L−1, pH 9.0) with stirring. Meanwhile, 100 mg DDTC-modified microspheres were dispersed in 5 mL Tris–HCl buffer solution (0.05 mmol L−1, pH 9.0) by ultrasonic vibration under conditions of avoidance of light. Then, the solutions were mixed together in a 50 mL quartz flask and purged with nitrogen for 30 min. Afterwards, the pre-polymer solution was kept at 0 °C for the self-assembly of TP5 and [VACMIM]Cl for three hours. Finally, ultraviolet irradiation from a high-pressure mercury lamp (300 W, 365 nm) emitting at 365 nm was applied to the mixture for three hours to obtain the grafting layer. The distance between the UV lamp and the flask was fixed at 10 cm. After polymerization, polymerized microspheres were collected, filtered and washed. Non-imprinting microspheres (NIMs) were synthesized under conditions identical to those used for the SMIMs except for the absence of template molecules.2.7 Removal of template molecules
After being irradiated for 3 h, the obtained polymers were washed with distilled water and separated by centrifugation at 5000 rpm several times to remove entrapped TP5 molecules and unreacted monomers. Afterwards, a 10% aqueous solution of acetic acid and distilled water were used to remove embedded template molecules and residual acetic acid, respectively. Complete removal was detected by a UV-2550 spectrophotometer at 276 nm. Then, the polymers were dried in a vacuum oven at 45 °C for 24 h.2.8 Adsorption, recognition and reusability experiments
To determine the adsorption capacities of SMIMs and NIMs, static adsorption experiments were carried out. In a typical procedure, 10.0 mg dried microspheres were dispersed in a 10 mL solution of TP5 in Tris–HCl buffer (pH = 9.0) with a certain concentration and were then shaken for a period of time at 25 °C. After separation by centrifugation, the concentration of TP5 in the supernatant was measured with a UV-vis spectrophotometer.The adsorption capacity (Qe) of the SMIMs (NIMs) for TP5, which was calculated on the basis of the difference in the TP5 concentration before and after adsorption, was calculated by the following eqn (1):
 | (1) |
The imprinting factor (IF) α was defined as representing the specificity of microspheres for TP5. It was calculated by the following eqn (2):
 | (2) |
The selectivity coefficient (SC) β was defined as representing the specific selectivity for analogues of the template molecule TP5.55 A higher value of the SC indicates better specific recognition and a better imprinting effect. The SC is defined by the following eqn (3):
 | (3) |
Reusability is an important property of imprinting polymers in practical applications. Reusability indicates the stability of polymers, which should be investigated. Adsorption–desorption cycles were carried out with the same batches of SMIMs. The loss of adsorption capacity after several adsorption–desorption cycles was used to determine the reusability of SMIMs.
3. Results and discussion
3.1 Preparation of P(EGDMA-CMS) microspheres
P(EGDMA-CMS) microspheres were synthesized via dispersion polymerization and used as the supporting material to prepare SMIMs. A modified approach for synthesizing microspheres developed by Du et al.52 was employed here and p-CMS was chosen as the copolymerization monomer in order to obtain surface functional groups for the further modification of iniferter groups. Here, the functional group benzyl chloride was introduced by the copolymerization of PEGDMA, EGDMA and p-CMS. Considering that the density of iniferter groups is crucial for the grafting process, more benzyl chloride groups should be generated at the surface of the P(EGDMA-CMS) microspheres. As a result, increasing the concentration of benzyl chloride groups on the surface of the microspheres is of great importance. There are two strategies that can be chosen in the process of synthesizing P(EGDMA-CMS) microspheres. One comprises the addition of p-CMS to a mixture of the other two monomers when polymerization is initiated. The other comprises the addition of p-CMS during the polymerization of PEGDMA and EGDMA after a period of time. As shown in Table 1 for the elemental analysis (EA), the concentrations of chlorine that resulted indicated that the latter method (4.61%) was better than the former method (0.84%) owing to the higher chlorine concentration. The reason for this may be that p-CMS is embedded when it is added at the same time as the other components, which results in a lower surface concentration of chlorine.| Sample | Elemental concentration (%) | ||||
|---|---|---|---|---|---|
| C | O | N | S | Cl | |
| a In the experiments, the time of the addition of p-CMS was 60 min when P(EGDMA-CMS) microspheres were synthesized.b In the experiments, the time of the addition of p-CMS was 0 min when P(EGDMA-CMS) microspheres were synthesized. | |||||
| P(EGDMA-CMS)a | 76.05 | 19.34 | 0 | 0 | 4.61 |
| P(EGDMA-CMS)b | 72.19 | 26.97 | 0 | 0 | 0.84 |
| P(EGDMA-CMS)@DDTC | 75.81 | 17.58 | 1.02 | 5.59 | 0 |
| SMIMs | 73.27 | 21.78 | 2.03 | 2.92 | 0 |
| NIMs | 73.50 | 23.61 | 1.25 | 1.64 | 0 |
3.2 Iniferter immobilization on P(EGDMA-CMS) microspheres
In order to immobilize the iniferter, a coupling reaction between the benzyl chloride groups of P(EGDMA-CMS) microspheres and DDTC was employed in dark conditions using ethanol as a solvent. The advantages of an immobilized iniferter were demonstrated. One is the stability of the dithiocarbamate radical and another is that the iniferter enables efficient control of the grafting process to control the thickness of the imprinting layer via the ultraviolet irradiation time.The iniferter immobilization strategy and surface imprinting technique were employed together here to solve the problems that occur in biomolecule imprinting processes. On the one hand, control of the thickness of the imprinting layer could overcome difficulties in mass transfer and the removal of template molecules. On the other hand, using ultraviolet irradiation instead of thermal initiation could avoid the adverse effects of using thermal irradiation in the imprinting process.
Although the coupling reaction can proceed to completion, the reaction conversion might be influenced by the reaction time. The reaction time was considered in order to obtain a higher iniferter density for better control of the surface layer. Qin et al.56 found that the atomic composition of N for MCP beads@DDTC increased slowly when the reaction time exceeded 24 h, that is to say, the reaction could be complete after 24 h; thus, the reaction time for modification was chosen to be 24 h in this study.
3.3 Characterization of P(EGDMA-CMS) and DDTC-modified microspheres
In order to confirm the success of each step during the reaction process, FT-IR and XPS were employed to give sufficient evidence. The shapes and morphologies were observed by SEM and TEM. EA was used as an auxiliary method of analysis to confirm the success of the reaction via changes in the concentration of different elements. The detailed results of the analysis were as follows.FT-IR measurements were performed to confirm the presence of C–Cl bonds and C–S bonds in the steps of synthesizing P(EGDMA-CMS) microspheres and coupling DDTC to the surface of microspheres, respectively. FT-IR spectra of all the samples in the region of 4000–400 cm−1 were obtained by the KBr pellet method with a Tensor 27 FTIR spectrometer (Bruker). The FT-IR spectra of P(EGDMA-CMS) microspheres and iniferter-modified microspheres are shown in Fig. 4a and b, respectively. For all the samples, a characteristic peak at 1727 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of carboxylic acid) and a peak at 1141 cm−1 (C–O stretching vibrations of the ester) were observed.57 Peaks at 2984 cm−1 (–CH3 stretching vibrations) and 2947 cm−1 (–CH2– stretching vibrations) were also observed. For the P(EGDMA-CMS) microspheres, the absorption at 676 cm−1 displayed the intensity of a characteristic C–Cl peak,58 which was initially present owing to vinylbenzyl chloride. For the sample of P(EGDMA-CMS)@DDTC, the disappearance of the C–Cl peak, as well as the appearance of peaks for C
O stretching vibrations of carboxylic acid) and a peak at 1141 cm−1 (C–O stretching vibrations of the ester) were observed.57 Peaks at 2984 cm−1 (–CH3 stretching vibrations) and 2947 cm−1 (–CH2– stretching vibrations) were also observed. For the P(EGDMA-CMS) microspheres, the absorption at 676 cm−1 displayed the intensity of a characteristic C–Cl peak,58 which was initially present owing to vinylbenzyl chloride. For the sample of P(EGDMA-CMS)@DDTC, the disappearance of the C–Cl peak, as well as the appearance of peaks for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds at 1210 cm−1 and C–S bonds at 712 cm−1, confirmed the successful immobilization of DDTC on the microspheres.59
S bonds at 1210 cm−1 and C–S bonds at 712 cm−1, confirmed the successful immobilization of DDTC on the microspheres.59
 | ||
| Fig. 4 FT-IR spectra of P(EGDMA-CMS) microspheres (a), DDTC-modified microspheres (b), SMIMs (c) and NIMs (d). | ||
XPS was used here to confirm the chemical composition of the surfaces at every step. For the sample of P(EGDMA-CMS), characteristic signals of O 1s (531.7 eV), C 1s (284.3 eV) and Cl 2p (199.8 eV) can be observed in the wide-scan spectrum, as shown in Fig. 5a. In the survey scan XPS spectrum of DDTC-modified P(EGDMA-CMS) microspheres (Fig. 5a), the Cl 2p peak at 199.7 eV disappeared and a S 2p peak at about 163 eV appeared in comparison to the spectrum of the sample of P(EGDMA-CMS) microspheres (Fig. 5a), which confirmed the successful coupling reaction between DDTC and P(EGDMA-CMS) microspheres. For the sample of P(EGDMA-CMS)@DDTC, the appearance of a N 1s peak (C–N–CH2CH3) at 399.7 eV also provided strong evidence for the success of the coupling reaction. The detailed S 2p spectrum was fitted to two doublets, which further confirmed the presence of N,N-diethyldithiocarbamyl groups. These two S 2p doublets may be readily explained. Typically, S 2p was fitted with a fixed branching ratio of the doublets of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The detailed S 2p doublets were recorded at 163.0 eV and 164.4 eV, which were attributed to S 2p3/2 (C–S single bonds) and S 2p1/2 (C
1. The detailed S 2p doublets were recorded at 163.0 eV and 164.4 eV, which were attributed to S 2p3/2 (C–S single bonds) and S 2p1/2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bonds), respectively. However, the area ratio of 1
S double bonds), respectively. However, the area ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and the difference in binding energy of ∼1.2 eV between the S 2p3/2 and S 2p1/2 components of the S 2p line confirmed the presence of N,N-diethyldithiocarbamyl groups.60 Moreover, the presence of a S 2s peak at 225.0 eV was accompanied by the occurrence of a S 2p peak at 164.0 eV. As shown in Fig. 6d, the C 1s high-resolution scan of the sample of DDTC-modified microspheres could be fitted to six peaks with binding energies of 284.6, 285.2, 285.7, 286.1, 287.2 and 288.7 eV, which were attributed to aliphatic carbon (C–H/C–C), C–S, C–N, ether (C–O), C
2 and the difference in binding energy of ∼1.2 eV between the S 2p3/2 and S 2p1/2 components of the S 2p line confirmed the presence of N,N-diethyldithiocarbamyl groups.60 Moreover, the presence of a S 2s peak at 225.0 eV was accompanied by the occurrence of a S 2p peak at 164.0 eV. As shown in Fig. 6d, the C 1s high-resolution scan of the sample of DDTC-modified microspheres could be fitted to six peaks with binding energies of 284.6, 285.2, 285.7, 286.1, 287.2 and 288.7 eV, which were attributed to aliphatic carbon (C–H/C–C), C–S, C–N, ether (C–O), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and ester carbon (O–C
S and ester carbon (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively. Moreover, the O 1s high-resolution scan could be fitted to two peaks with binding energies of 531.9 and 533.3 eV, which were attributed to ether oxygen (C–O) and ester oxygen (O–C
O), respectively. Moreover, the O 1s high-resolution scan could be fitted to two peaks with binding energies of 531.9 and 533.3 eV, which were attributed to ether oxygen (C–O) and ester oxygen (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively. These results are fully consistent with those of the FT-IR analysis.
O), respectively. These results are fully consistent with those of the FT-IR analysis.
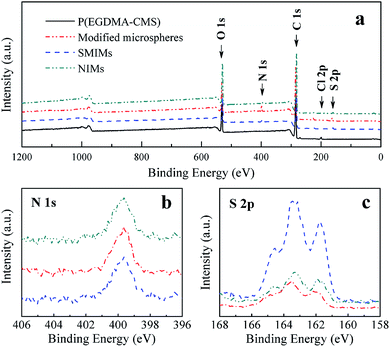 | ||
| Fig. 5 (a) XPS wide-scan survey spectra of P(EGDMA-CMS), P(EGDMA-CMS)@DDTC, SMIMs, and NIMs; (b) high-resolution spectrum of N 1s; (c) high-resolution spectrum of S 2p. | ||
 | ||
| Fig. 6 Curve fitting of high-resolution spectra for C 1s (a), O 1s (b), and Cl 2p (c) for P(EGDMA-CMS) microspheres and C 1s (d), N 1s (e) and S 2p (f) for DDTC-modified microspheres. | ||
EA is used as an auxiliary method for the identification of a successful reaction or modification process. For the sample of P(EGDMA-CMS), three elements were present, namely, C, O and Cl and their concentrations by mass were 76.05%, 19.34% and 4.61%, respectively. After modification by DDTC, the obvious changes were the disappearance of Cl and the appearance of N (1.02%) and S (5.59%). Moreover, the EA results showed that the elemental concentration by mass of O underwent a decrease of ∼2%. This may be due to the fact that the introduction of the iniferter group decreased the relative concentration of oxygen. In addition, oxygen atoms were sheltered from other atoms so that X-rays could not strike them and excite photoelectrons. As a result, the signal of oxygen could not be detected and the concentration by mass of O that was recorded decreased.
On the basis of the above-mentioned discussions, the successful synthesis of P(EGDMA-CMS)@DDTC by a coupling reaction under dark conditions could be confirmed by full characterization by XPS, FT-IR and EA. Then, the grafting process under ultraviolet irradiation could be carried out based on the DDTC-modified microspheres.
3.4 Synthesis and characterization of surface imprinting layer
The process of imprinting is illustrated in Scheme 1 and combined an iniferter immobilization strategy and a surface imprinting technique. The reasons why [VACMIM]Cl was synthesized and used as a functional monomer are stated as follows. Firstly, biomolecules are water-soluble, which leads to limitations on functional monomers, and an ionic liquid could widen the range of functional monomers that can be used in the aqueous phase. Secondly, an ionic liquid can help dissolve the template molecule TP5 in Tris–HCl buffer solution. Thirdly, the multiple interactions that occur between an ionic liquid and TP5 could not only increase the adsorption capacity but also enhance the specific recognition properties.XPS, FT-IR and EA were employed to fully characterize the core–shell SMIMs. The successful synthesis of the surface imprinting layer was confirmed by XPS. For both SMIMs and NIMs, characteristic signals of O 1s (530.1 eV), C 1s (284.3 eV), N 1s (397.0 eV) and S 2p (164.0 eV) can be observed in the XPS wide-scan spectrum. Notable changes in the N 1s peak in comparison with that due to the iniferter immobilization process were observed, which included changes in the shape and intensity of the peak owing to new types of nitrogen atoms introduced by [VACMIM]Cl and MBA. The changes indicated that the surface imprinting layer was successfully synthesized and core–shell structure polymers were obtained by iniferter-induced radical polymerization. With the aim of investigating the polymerization reaction between [VACMIM]Cl and MBA, high-resolution XPS spectra were recorded and curve fitting of the N 1s peak was performed for both SMIMs and NIMs. As shown in Fig. 7, the N 1s high-resolution XPS scan could be fitted to three peaks with binding energies of 399.5, 400.3 and 399.9 eV for the sample of SMIMs, which were attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–N (imidazole ring) and –NH2 (amino)/C–N–Et2/(C–O)–(N–H), respectively. For the sample of P(EGDMA-CMS)@DDTC, the N 1s peak at 399.7 eV was attributed to C–N–Et2. After grafting, there should be five types of nitrogen atom in total, namely, C–N–Et2, –(C–O)–(N–H), –NH2 and two types of nitrogen atom in the imidazole ring, respectively. There is evidence that the binding energies (BE) of –(C–O)–(N–H) and –NH2 have the same value of 399.9 eV.61 In addition, the value of the BE of C–N–Et2 was so close to that of –NH2 (399.9 eV) that the signals could not be distinguished when curve fitting was performed. As a result, three types of N 1s signal overlapped at the same position and formed a single signal at 399.9 eV. The curve fitting for SMIMs was reasonable from the aforementioned analysis. Some characteristic peaks of SMIMs and NIMs at 1503, 1420, 680, and 1650 cm−1 were observed in the FT-IR spectra, which indicated that VI and amino groups of the ionic liquid [VACMIM]Cl were attached to the structure of the microspheres. In addition, the peaks at 1210 cm−1 (C
N, C–N (imidazole ring) and –NH2 (amino)/C–N–Et2/(C–O)–(N–H), respectively. For the sample of P(EGDMA-CMS)@DDTC, the N 1s peak at 399.7 eV was attributed to C–N–Et2. After grafting, there should be five types of nitrogen atom in total, namely, C–N–Et2, –(C–O)–(N–H), –NH2 and two types of nitrogen atom in the imidazole ring, respectively. There is evidence that the binding energies (BE) of –(C–O)–(N–H) and –NH2 have the same value of 399.9 eV.61 In addition, the value of the BE of C–N–Et2 was so close to that of –NH2 (399.9 eV) that the signals could not be distinguished when curve fitting was performed. As a result, three types of N 1s signal overlapped at the same position and formed a single signal at 399.9 eV. The curve fitting for SMIMs was reasonable from the aforementioned analysis. Some characteristic peaks of SMIMs and NIMs at 1503, 1420, 680, and 1650 cm−1 were observed in the FT-IR spectra, which indicated that VI and amino groups of the ionic liquid [VACMIM]Cl were attached to the structure of the microspheres. In addition, the peaks at 1210 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bonds), 712 cm−1 (C–S single bonds) and 1728 cm−1 (C
S double bonds), 712 cm−1 (C–S single bonds) and 1728 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of carboxylic acid) are absorption signals of some characteristic functional groups that exist in the structure of polymers. As shown in Table 1, EA analysis of SMIMs showed that the atomic concentration of N underwent a notable change of 1.01% in comparison with that in DDTC-modified microspheres, which suggested that [VACMIM]Cl and MBA were introduced and the surface imprinting layer was formed. In addition, the atomic concentration of S underwent an obvious decline after the surface layer grafting process, which indicated that some sulfur atoms were embedded after the grafting process. The results of the FT-IR analysis and EA analysis were in good agreement with those of the XPS analysis shown above.
O stretching vibrations of carboxylic acid) are absorption signals of some characteristic functional groups that exist in the structure of polymers. As shown in Table 1, EA analysis of SMIMs showed that the atomic concentration of N underwent a notable change of 1.01% in comparison with that in DDTC-modified microspheres, which suggested that [VACMIM]Cl and MBA were introduced and the surface imprinting layer was formed. In addition, the atomic concentration of S underwent an obvious decline after the surface layer grafting process, which indicated that some sulfur atoms were embedded after the grafting process. The results of the FT-IR analysis and EA analysis were in good agreement with those of the XPS analysis shown above.
SEM was employed to investigate the sizes and morphologies of the P(EGDMA-CMS) microspheres, SMIMs and NIMs. As shown in Fig. 8a and b, the P(EGDMA-CMS) microspheres exhibited a uniform spherical shape with a diameter of 554 ± 21 nm. The uniform size of the microspheres favored a reduction in interference when the adsorption properties were investigated and gave rise to a uniform grafting layer as well. Moreover, TEM was employed to observe the core–shell structure and confirm the successful preparation of the surface imprinting layer. TEM images of P(EGDMA-CMS) and SMIMs are shown in Fig. 8c and d, respectively. It was found that the SMIMs that were obtained exhibited an obvious core–shell structure after the surface grafting process, which indicated that a shell layer with a thickness of ∼35 nm was coated on the surface of P(EGDMA-CMS)@DDTC. The results suggested that the shell layer would be thin, which could be beneficial for mass transport between the surface of SMIMs and the buffer solution.
 | ||
| Fig. 8 SEM images of P(EGDMA-CMS) microspheres (a, b) and TEM images of P(EGDMA-CMS) microspheres (c) and SMIMs (d). | ||
In order to investigate the thickness of the imprinting layer prepared via iniferter-induced radical polymerization, statistical data for the thickness of the imprinting layer were gathered from TEM micrographs of surface imprinting microspheres. Thirty sample thicknesses were selected randomly and the average thickness was 35.3 nm with a standard deviation (SD) of 3.5 nm. The statistical results showed that 70% of the thickness values were in the range from 30 nm to 37 nm. The results indicated that the imprinting layer was well controlled via CRP, which suggested that a relatively uniform shell layer was formed and reflected the controllability of the iniferter.
3.5 Adsorption isotherm study
In order to investigate the binding properties of SMIMs with TP5, adsorption isotherm experiments were conducted in solutions of TP5 in a buffer with concentrations ranging from 0.05 to 0.50 mg mL−1. As shown in Fig. 9a, the adsorption isotherm process included a linear stage of slight increases at low concentrations, a stage of rapid increases at intermediate concentrations and a saturation stage. The adsorption capacity (Qe) for TP5 of the SMIMs increased rapidly with an increase in the initial concentration of TP5 from 0.05 to 0.10 mg mL−1, increased slightly from 0.10 mg mL−1 to 0.3 mg mL−1 and reached an equilibrium above 0.4 mg mL−1. The adsorption capacity of SMIMs (27.6 mg g−1) was about 2.33 times that of NIMs at a concentration of 0.3 mg mL−1. It was shown that SMIMs had a higher binding capacity than that of NIMs. The results indicated that SMIMs had more effective binding sites than NIMs. The imprinting effect was the reason why the effective binding sites of SMIMs were in large quantities and evenly distributed. In addition, SMIMs displayed better chemical matching with the template peptide than that of NIMs. However, NIMs had no binding sites owing to the random arrangements of functional monomers and non-specific adsorption had a predominant effect. | ||
| Fig. 9 (a) Adsorption isotherms of SMIMs and NIMs in the concentration range from 0.05 to 0.50 mg mL−1 at 25 °C. (b) Scatchard plot of SMIMs for TP5 as the template: Ce/Qe versus Ce. | ||
A maximum adsorption capacity (27.6 mg g−1) and imprinting factor (IF = 2.33) were observed when the concentration of TP5 was 0.3 mg mL−1. In comparison with a previous work by our research group,8 in which MAA was selected as the functional monomer by employing fragment imprinting, the adsorption capacity underwent an obvious improvement at a similar level of the IF (IF = 2.06). This may be because the single interaction that took place between SMIMs and the template could not ensure adsorption and selectivity at the same time. In addition, fragment imprinting did not imprint the precise structure of the template molecules, so that unsatisfactory adsorption properties were inevitable. Furthermore, the idea that the multiple interactions provided by the ionic liquid could enhance the adsorption properties of SMIMs was confirmed in some respects. The conclusions were in good agreement with research by Du et al.52
The Langmuir isotherm model was used here to analyze the adsorption isotherm data and can be expressed as eqn (4):
 | (4) |
As shown in Fig. 9b, the Langmuir linear regression equation of SMIMs is Ce/Qe = 0.01691Ce + 0.00581 (R2 = 0.9943). According to the correlation coefficient R2 = 0.9943, we could draw the conclusion that the Langmuir isotherm model gives a good fit for the set of data in the range of concentrations, which suggests that monolayer adsorption occurred on the surface of SMIMs. The two parameters, namely, KL and Qmax, were calculated from the intercept and slope of the Langmuir linear regression equation and were estimated to be 2.9105 mL mg−1 and 59.1 mg g−1, respectively.
Scatchard analysis was carried out to investigate the binding properties of the SMIMs with TP5. The saturation binding data were plotted according to the Scatchard equation to estimate the binding properties of the SMIMs with TP5. Furthermore, the Scatchard plot could indicate how many types of binding site were present in the SMIMs. In general, if a Scatchard plot can be fitted to only one line, this indicates that there is one type of binding site present in the SMIMs. If they can be fitted to two or more lines, this indicates that there are various types of binding site present.
Scatchard analysis of the SMIMs was performed based on data from adsorption experiments. From the results of the analysis shown in Fig. 9b, we found that the Scatchard plot was one straight line, which indicated that there was one type of binding site and the presence of binding sites in SMIMs was actually confirmed.
3.6 Adsorption kinetics study
The kinetics of the adsorption of a 0.3 mg mL−1 solution of TP5 in a buffer onto SMIMs and NIMs are illustrated in Fig. 10. It was found that SMIMs displayed a higher binding capacity for TP5 than that of NIMs in the first 45 min and even achieved 68% of their equilibrium adsorption capacity, and the binding capacity changed slowly over the next 75 min. These results indicated that the binding sites were occupied by the TP5 template at a rapid rate, which suggested that the binding sites were formed on the surface of the SMIMs. The process took about 120 min to reach adsorption equilibrium. The adsorption kinetics underwent different stages in the whole adsorption process. At the beginning of the adsorption process, there were many empty binding sites present on the surface of the SMIMs, which enabled a rapid adsorption process with low mass transfer resistance. After a period of time, the adsorption rate slowed down because most binding sites were occupied and there were then insufficient binding sites. In contrast, NIMs displayed a low affinity for TP5 because they possessed few or no efficient binding sites, so that non-specific adsorption was predominant in the adsorption process.Pseudo-first-order and pseudo-second-order kinetic models were employed to fit the kinetics experimental data to further investigate the adsorption kinetics of TP5. The two kinds of model are shown as follows.
The expression for the pseudo-first-order equation is:
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − K1t Qe − K1t
| (5) |
The expression for the pseudo-second-order equation is:
 | (6) |
In general, in the pseudo-first-order model it is considered that the rate of occupation of adsorption sites is proportional to the number of unoccupied sites, whereas in the pseudo-second-order kinetic model it is assumed that the adsorption rate is controlled by chemical adsorption.62 The parameters and a linear regression analysis of the two types of model are given in Table 2 and Fig. 11.
| Microsphere | Qe,exp (mg g−1) | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|---|
| Qe,cal (mg g−1) | K1 (min−1) | R2 | Qe,cal (mg g−1) | K2 (g mg−1 min−1) | R2 | ||
| SMIMs | 27.5 | 32.5 | 0.0277 | 0.9605 | 35.7 | 6.02 × 10−4 | 0.9950 |
| NIMs | 12.7 | 15.9 | 0.0339 | 0.9898 | 16.7 | 1.35 × 10−3 | 0.9492 |
 | ||
| Fig. 11 Pseudo-first-order kinetics (a) and pseudo-second-order kinetics (b) models of SMIMs and NIMs at 25 °C. | ||
The principles of selecting a best-fit model are based on the linear regression correlation coefficient (R2) and the theoretical equilibrium adsorption capacity (Qe(cal)). The pseudo-second-order rate equation for the adsorption of TP5 agreed well with the data with a high correlation coefficient (R2 = 0.9950). In addition, the theoretical value (Qe(cal)) was close to the experimental value (Qe(exp)) to a certain extent. As a result, we could conclude that the pseudo-second-order kinetic model provided a good correlation for the SMIMs instead of the pseudo-first-order kinetic model for the adsorption of TP5 in this study and the adsorption process was chemical in nature, which suggested that binding sites were actually formed and chemical adsorption played the predominant role in the adsorption process.
In the adsorption buffer solution (pH = 9.0), positively charged imidazole rings at the binding sites of SMIMs attracted negatively charged carboxyl groups in TP5. The non-directional electrostatic interaction that occurred between SMIMs and TP5 and the thin shell layer are the reasons why the SMIMs displayed a high adsorption capacity.52,63 Furthermore, hydrogen bonds formed between the terminal amino groups of [VACMIM]Cl, terminal carboxyl groups that were not ionized and amino groups, which were directional and helped improve the selectivity of adsorption. A synergistic effect was fully exhibited in the adsorption process, which was why SMIMs exhibited good adsorption capacity and high selectivity at the same time. The binding energies and directionality of non-covalent interactions are shown in Table 3. Among these interactions, electrostatic interactions have high binding energies but are non-directional interactions, which caused the SMIMs to exhibit high adsorption capacity but led to little improvement in selectivity, whereas hydrogen bonding is relatively weak but is a directional interaction, which caused hydrogen bonding to play a predominant role in the improvement in selectivity. In addition, there might be some weak interactions such as π−π stacking and hydrophobic interactions. These interactions could not be ignored because they might play important roles in the adsorption process.57 In the adsorption process, binding sites in SMIMs were occupied by TP5 gradually as time passed, which caused a decrease in the concentration of TP5 in the buffer solution. Specific adsorption might have occurred at the surface of SMIMs, although it played a minor role in the adsorption of TP5. Specific adsorption caused by binding sites was the predominant form for SMIMs and their adsorption capacity could be high, whereas for NIMs TP5 molecules were mainly adsorbed on the surface, which suggested that non-specific adsorption played the predominant role in the adsorption process. This was because there were no or fewer binding sites formed in the imprinting process because no template molecules were added for guidance. Even if there were binding sites in NIMs, [VACMIM]Cl had a random arrangement and effective binding sites could not be formed. As a result, NIMs displayed low adsorption capacity and reached a rapid equilibrium.
| Type of binding interaction | Binding energy (kcal mol−1) | Directionality | |
|---|---|---|---|
| Ion–dipole | Ion–ion | 20–80 | Non-directional |
| Ion–dipole | 12–50 | ||
| Dipole–dipole | 1–10 | ||
| Coordinate bonding | 20–50 | Directional | |
| Hydrogen bonding | 1–30 | Directional | |
| π–π stacking | 0–12 | — | |
| van der Waals interactions | 0–1.5 | Non-directional | |
3.7 Determination of selectivity of SMIMs
To further investigate the selectivity properties of surface imprinting microspheres, competitive adsorption experiments were carried out using IPH as the competitor molecule. IPH (Mw: 716.8, pI 3.30, H-Val-Glu-Pro-Ile-Pro-Tyr-OH, Fig. 1b), which has two amino acid residues in common with TP5 (Mw: 679.8, pI 8.59, H-Arg-Lys-Asp-Val-Tyr-OH, Fig. 1a), was chosen as the competitor peptide. Competitive adsorption experiments on SMIMs and NIMs were carried out in a solution mixture of TP5 and IPH with a total concentration of 0.30 mg mL−1, which indicated that the molar concentrations of TP5 and IPH were both 0.215 μmol mL−1. As shown in Fig. 12 and Table 4, the specific adsorption capacity of SMIMs for TP5 was 12.9 mg mL−1, which was much higher than that for the competitor peptide IPH. Moreover, the values of the IF (α) and SC (β) were 2.48 and 2.18, respectively, which indicated that the SMIMs exhibited obvious adsorption selectivity for TP5. In contrast, the imprinting factor for IPH was only 1.14. It appeared that the surface imprinting layer was successfully synthesized and the selective binding sites with chemical matching properties for TP5 were formed in another way. The ordered arrangement of chemical groups on the surface of SMIMs can match well with the template molecule TP5 instead of the analogue IPH, which was why SMIMs displayed specific recognition properties for TP5.| Peptide | QSMIMs (mg g−1) | QNIMs (mg g−1) | IF | SC |
|---|---|---|---|---|
| a In this experiment, 30 mg SMIMs or NIMs was incubated in a mixture of TP5 and IPH at a total concentration of 0.30 mg mL−1 for 180 min. | ||||
| TP5 | 12.9 | 5.2 | 2.48 | — |
| IPH | 4.8 | 4.2 | 1.14 | 2.18 |
3.8 Reusability and reproducibility of imprinting polymers
Reusability is one of the most important properties of imprinting polymers used as separating materials in practical applications. Adsorption–desorption experiments were performed to determine the stability of SMIMs. Samples of the same batch including SMIMs and NIMs were used in continuous adsorption–desorption experiments for three cycles. As shown in Table 5, the adsorption capacities of SMIMs gradually decreased over their time in use for different concentration. When the three cycles of adsorption–desorption experiments had finished, the SMIMs only lost 7.6% and 11.4% of their adsorption capacity for TP5 at concentrations of 0.05 and 0.30 mg g−1, respectively, which indicated that the SMIMs were relatively stable and could maintain their adsorption capacity after a multi-use process. The loss of adsorption capacity may be caused by disruption of binding sites when the template was removed and the decrease in effective binding sites. The results indicated that the imprinting polymers had satisfactory reusability and potential applications in practice.| Initial TP5 concentration | Adsorption capacity (mg g−1) | ||
|---|---|---|---|
| 1st | 2nd | 3rd | |
| 0.05 mg mL−1 | 7.9 ± 1.2 | 7.5 ± 1.0 | 7.3 ± 0.6 |
| 0.30 mg mL−1 | 26.3 ± 1.8 | 25.1 ± 1.6 | 23.3 ± 3.0 |
4. Conclusions
We have synthesized core–shell SMIMs using iniferter-induced radical polymerization and the ‘grafting from’ approach for the rapid recognition of TP5. A surface imprinting technique and an iniferter immobilization strategy were combined to achieve better control of the imprinting layer under irradiation by ultraviolet light. A novel ionic liquid with a C–C double bond and a terminal amino group, namely, [VACMIM]Cl, was synthesized and used as a functional monomer. Multiple interactions between the ionic liquid and template molecules including π–π stacking interactions, hydrogen bonding and electrostatic interactions played important roles in the improvement of the adsorption properties of SMIMs. The imprinting polymers that were obtained displayed high adsorption capacity (Qmax = 32.0 mg g−1), good selectivity (SC = 2.18), satisfactory reusability and rapid binding equilibrium (tequ = 120 min) with TP5. We believe that the SMIMs have the potential for applications in the recognition and separation of biomolecules, as well as chemical sensors for highly sensitive detection, in the future.Acknowledgements
The authors are grateful for the financial support of the National Natural Science Foundation of China (Grant No. 51433008) and the Graduate Starting Seed Fund of Northwestern Polytechnical University (Grant No. Z2016034).References
- C. Wu, M. Zhang, Z. Zhang, K. W. Wan, W. Ahmed, D. A. Phoenix, A. M. A. Elhissi and X. Sun, Mol. Pharm., 2014, 11, 3371–3377 CrossRef CAS PubMed.
- R. Colle, T. Ceschia, A. Colatutto and F. Biffoni, Curr. Ther. Res., 1988, 44, 1045–1049 Search PubMed.
- M. G. Bernengo, G. C. Doveil, M. Meregalli, A. Appino and R. Massobrio, Br. J. Dermatol., 1988, 119, 207–221 CrossRef CAS PubMed.
- A. L. Weaver, M. A. Churchill and A. J. Jacobs, Clin. Res., 1983, 31, 807 Search PubMed.
- E. Faist, W. Ertel, B. Salmen, A. Weiler, C. Ressel, K. Bolla and G. Heberer, Arch. Surg., 1988, 123, 1449–1453 CrossRef CAS PubMed.
- E. Wolf, S. Milazzo, K. Boehm, M. Zwahlen and M. Horneber, The Cochrane Library, 2011 Search PubMed.
- X. Jin, Y. Xu, J. Shen, Q. Ping, Z. Su and W. You, Carbohydr. Polym., 2011, 86, 51–57 CrossRef CAS.
- L. X. Guo, X. L. Hu, P. Guan, C. B. Du, D. Wang, D. M. Song, X. M. Gao and R. Y. Song, Appl. Surf. Sci., 2015, 357, 1490–1498 CrossRef CAS.
- C. L. Wang, X. L. Hu, P. Guan, L. W. Qian, D. F. Wu and J. Li, Sep. Sci. Technol., 2015, 50, 1768–1775 CrossRef CAS.
- M. T. H. Hearn, C. A. Bishop, W. S. Hancock, D. R. K. Harding and G. D. Reynolds, J. Liq. Chromatogr., 1979, 2, 1–21 CrossRef CAS.
- R. Gutiérrez-Climente, A. Gómez-Caballero, M. Halhalli, B. Sellergren, M. A. Goicolea and R. J. Barrio, J. Mol. Recognit., 2016, 29, 106–114 CrossRef PubMed.
- C. Allender and K. Mosbach, Biosens. Bioelectron., 2009, 25, 539–542 CrossRef CAS PubMed.
- L. X. Chen, S. F. Xu and J. H. Li, Chem. Soc. Rev., 2011, 40, 2922–2942 RSC.
- Molecular Imprinting: Principles and Applications of Micro- and Nanostructure Polymers, ed. L. Ye, CRC Press, Singapore, 2013, pp. 105–160 Search PubMed.
- H. Y. Shen, B. W. Liu, Q. Xiang, C. C. Wang and S. Q. Mao, RSC Adv., 2016, 6, 81330–81340 RSC.
- T. H. Jiang, L. X. Zhao, B. L. Chu, Q. Z. Feng, W. Yan and J. M. Lin, Talanta, 2009, 78, 442–447 CrossRef CAS PubMed.
- W. Huang, P. Xu, W. Yang and W. Xu, RSC Adv., 2016, 6, 74734–74741 RSC.
- X. T. Shen, C. G. Xu and L. Ye, Ind. Eng. Chem. Res., 2012, 52, 13890–13899 CrossRef.
- D. Cai, L. Ren, H. Zhao, C. Xu, L. Zhang, Y. Yu, H. Z. Wang, Y. C. Lan, M. F. Roberts, J. H. Chuang, M. J. Naughton, Z. F. Ren and T. C. Chiles, Nat. Nanotechnol., 2010, 5, 597–601 CrossRef CAS PubMed.
- C. Y. Wang, A. Javadi, M. Ghaffari and S. Q. Gong, Biomaterials, 2010, 31, 4944–4951 CrossRef CAS PubMed.
- J. Yin, Y. Cui, G. Yang and H. Wang, Chem. Commun., 2010, 46, 7688–7690 RSC.
- M. Resmini, Anal. Bioanal. Chem., 2012, 402, 3021–3026 CrossRef CAS PubMed.
- E. Rodríguez, F. Navarro-Villoslada, E. Benito-Peña, M. D. Marazuela and M. C. Moreno-Bondi, Anal. Chem., 2011, 83, 2046–2055 CrossRef PubMed.
- X. Shen, L. Zhu, G. Liu, H. Tang, S. Liu and W. Li, New J. Chem., 2009, 33, 2278–2285 RSC.
- T. Zhou, L. Jørgensen, M. A. Mattebjerg, I. S. Chronakis and L. Ye, RSC Adv., 2014, 4, 30292–30299 RSC.
- T. W. Tan, Molecular Imprinting Technique and Its Application, Chemical Industry Press, Beijing, 2010, pp. 226–270 Search PubMed.
- C. Gonzato, M. Courty, P. Pasetto and K. Haupt, Adv. Funct. Mater., 2011, 21, 3947–3953 CrossRef CAS.
- N. Marchyk, J. Maximilien, S. Beyazit, K. Haupt and B. Tse Sum Bui, Nanoscale, 2014, 6, 2872–2878 RSC.
- H. Q. Zhang, Eur. Polym. J., 2013, 49, 579–600 CrossRef CAS.
- M. Bompar and K. Haupt, Aust. J. Chem., 2009, 62, 751–761 CrossRef.
- S. F. Xu, J. H. Li and L. X. Chen, J. Mater. Chem., 2011, 21, 4346–4351 RSC.
- K. Haupt, A. V. Linares, M. B. Bompart and T. S. Bui, Top. Curr. Chem., 2012, 325 Search PubMed.
- T. Otsu and Y. Masatoshi, Macromol. Rapid Commun., 1982, 3, 127–132 CrossRef CAS.
- T. Otsu, T. Matsunaga, T. Doi and A. Matsumoto, Eur. Polym. J., 1995, 31, 67–78 CrossRef CAS.
- S. Su, M. Zhang, B. Li, H. Zhang and X. Dong, Talanta, 2008, 76, 1141–1146 CrossRef CAS PubMed.
- N. Pérez-Moral and A. G. Mayes, Macromol. Rapid Commun., 2007, 28, 2170–2175 CrossRef.
- R. R. Chen, L. Qin, M. Jia, X. W. He and W. Y. Li, J. Membr. Sci., 2010, 363, 212–220 CrossRef CAS.
- H. Wang, X. Dong and M. Yang, TrAC, Trends Anal. Chem., 2012, 31, 96–108 CrossRef CAS.
- J. B. Pu, L. P. Wang, Y. F. Mo and Q. J. Xue, J. Colloid Interface Sci., 2011, 354, 858–865 CrossRef CAS PubMed.
- H. Y. Yan, S. T. Liu, M. M. Gao and N. Sun, J. Chromatogr. A, 2013, 1294, 10–16 CrossRef CAS PubMed.
- K. Tsunashima, E. Niwa, S. Kodama, M. Sugiya and Y. Ono, J. Phys. Chem. B, 2009, 113, 15870–15874 CrossRef CAS PubMed.
- Q. H. Zhang and J. M. Shreeve, Chem. Rev., 2014, 114, 10527–10574 CrossRef CAS PubMed.
- T. D. Ho, C. Zhang, L. W. Hantao and J. L. Anderson, Anal. Chem., 2013, 86, 262–285 CrossRef PubMed.
- N. Zhou, J. H. Li, H. Chen and L. X. Chen, Analyst, 2013, 138, 1091–1097 RSC.
- E. B. Fox, H. R. Colón-Mercado, Y. Chen and W. S. Winston Ho, ACS Symp. Ser., 2012, 1117, 129–143 CrossRef CAS.
- F. J. Chen, Z. Y. Song, J. Nie, G. W. Yu, Z. G. Li and M. Lee, RSC Adv., 2016, 6, 81877–81885 RSC.
- L. Vidal, J. Parshintsev, K. Hartonen, A. Canals and M. L. Riekkola, J. Chromatogr. A, 2012, 1226, 2–10 CrossRef CAS PubMed.
- Z. X. Xu, G. Z. Fang and S. Wang, Food Chem., 2010, 119, 845–850 CrossRef CAS.
- W. T. Bi, M. L. Tian and K. H. Row, J. Chromatogr. A, 2012, 1232, 37–42 CrossRef CAS PubMed.
- C. Y. He, Y. Y. Long, J. L. Pan, K. Li and F. Liu, Talanta, 2008, 74, 1126–1131 CrossRef CAS PubMed.
- K. Booker, M. C. Bowyer, C. I. Holdsworth and A. McCluskey, Chem. Commun., 2006, 16, 1730–1732 RSC.
- C. B. Du, X. L. Hu, P. Guan, L. X. Guo, L. W. Qian, R. Y. Song, J. Li and C. L. Wang, J. Mater. Chem. B, 2015, 3, 3044–3053 RSC.
- M. L. Tian, W. T. Bi and K. H. Row, Anal. Bioanal. Chem., 2011, 399, 2495–2502 CrossRef CAS PubMed.
- J. E. Lofgreen and G. A. Ozin, Chem. Soc. Rev., 2014, 43, 911–933 RSC.
- C. J. Zhang, Y. Z. Wang, Y. G. Zhou, J. X. Guo and Y. J. Liu, Anal. Methods, 2014, 6, 8584–8591 RSC.
- L. Qin, X. W. He, W. Zhang, W. Y. Li and Y. K. Zhang, J. Chromatogr. A, 2009, 1216, 807–814 CrossRef CAS PubMed.
- A. M. I. Ali and A. G. Mayes, Macromolecules, 2009, 43, 837–844 CrossRef.
- C. Kavaklı, S. Malcı, S. A. Tuncel and B. Salih, React. Funct. Polym., 2006, 66, 275–285 CrossRef.
- A. Bossi, M. J. Whitcombe, Y. Uludag, S. Fowler, I. Chianella, S. Subrahmanyam, I. Sanchez and S. A. Piletsky, Biosens. Bioelectron., 2010, 25, 2149–2155 CrossRef CAS PubMed.
- T. Kamra, S. Chaudhary, C. Xu, L. Montelius, J. Schnadt and L. Ye, J. Colloid Interface Sci., 2016, 461, 1–8 CrossRef CAS PubMed.
- V. Wiertz and P. Bertrand, Identification of the N-containing functionalities introduced at the surface of ammonia plasma treated carbon fibres by combined ToF-SIMS and XPS J, 1998 Search PubMed.
- Y. Liu, Z. C. Liu, J. Gao, J. D. Dai, J. Han, Y. Wang, J. M. Xie and Y. Yan, J. Hazard. Mater., 2011, 186, 197–205 CrossRef CAS PubMed.
- L. H. Zhang, N. R. Brostowitz, K. A. Cavicchi and R. A. Weiss, Macromol. React. Eng., 2014, 8, 81–99 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |




