Dieckol, an edible seaweed polyphenol, retards rotenone-induced neurotoxicity and α-synuclein aggregation in human dopaminergic neuronal cells†
Seon-Heui Cha*ab,
Soo-Jin Heoc,
You-Jin Jeond and
Sang Myun Park *abe
*abe
aDepartment of Pharmacology, Ajou University School of Medicine, 164, Worldcup-ro, Yeongtong-gu, Suwon, 16499, Korea. E-mail: sunnyday8109@gmail.com; sangmyun@ajou.ac.kr; Fax: +82-31-219-5069; Tel: +82-31-219-5067 Tel: +82-31-219-5063
bChronic Inflammatory Disease Research Center, Ajou University School of Medicine, Suwon, 16499, Korea
cJeju International Marine Science Center for Research & Education, Korea Institute of Ocean Science & Technology (KIOST), Jeju, 63349, Korea
dSchool of Marine Biomedical Sciences, Jeju National University, Jeju, 63243, Korea
eBK21 Plus Program, Department of Biomedical Sciences, Ajou University School of Medicine, Suwon, 16499, Korea
First published on 11th November 2016
Abstract
Dopaminergic neurons are particularly vulnerable to oxidative stress, which may initiate a cascade of intracellular toxic events that lead to protein aggregation and subsequent cell death, causing Parkinson's disease. Here, we investigate the neuroprotective effect of dieckol, which is a polyphenol isolated from an edible seaweed, Ecklonia cava, on rotenone-induced oxidative stress in SH-SY5Y cells, a human dopaminergic neuronal cell line. Dieckol was found to reduce intracellular reactive oxygen species (ROS) and cytochrome C release induced by treatment with rotenone. Consequently, dieckol reduced rotenone-induced cell death, and retarded rotenone-induced α-synuclein aggregation in α-synuclein-overexpressing SH-SY5Y cells. These results clearly indicate that dieckol possesses prominent antioxidant activity in dopaminergic neuronal cells preventing α-synuclein aggregation. Therefore, it could be a potential therapeutic agent for the prevention of neurodegenerative diseases such as Parkinson's disease.
1. Introduction
Parkinson's disease (PD) is a common neurodegenerative disease characterized by progressive degeneration of midbrain dopaminergic neurons, resulting in motor dysfunction.1 Although the pathogenesis of dopaminergic neuronal degeneration is still unclear, it has been demonstrated that several pathological processes such as protein aggregation,2 mitochondrial dysfunction,3 and oxidative stress4,5 play important roles in the pathogenesis of PD. Several epidemiological studies have revealed that certain pesticides including rotenone and paraquat and environmental factors related to pesticide exposure such as farming, rural living, and well water consumption have a strong correlation with an increased incidence of PD.6–8 Moreover, many pesticides cause mitochondrial dysfunction by inhibiting complex I of the respiratory chain.9–10 It has also been demonstrated that complex I inhibition results in an increased production of reactive oxygen species (ROS).9 Rotenone is a powerful and selective mitochondrial complex I inhibitor that initiates ROS production and cytochrome C release, causing apoptotic cell death.9 It also induces aggregation of the oxidized proteins.11,12 In particular, it can mimic most clinical features of PD such as progressive dopaminergic neuronal loss and Lewy body formation, indicating that rotenone neurotoxicity is a possible etiological factor in PD.11,13–15Seaweeds are composed of a variety of bioactive materials such as polysaccharides, pigments, minerals, peptides and polyphenols with valuable pharmaceutical and biomedical potential.16 In particular, brown seaweeds contain various biological benefits.17–21 The biological properties of a brown seaweed, Ecklonia cava (E. cava), are attributed to biologically-active secondary metabolites such as phlorotannins including dieckol.22–24 Phlorotannins exhibit a variety of biological properties, including antioxidative,25,26 anti-inflammatory,27 anti-allergenic,28 neuroprotective,29 and memory enhancing effects,30 and the improvement of sleep.31 Dieckol is a structurally powerful radical scavenger and the most abundant compound in phlorotannin extracts from brown seaweeds.25–31
In this study, we determine the protective effect of dieckol isolated from an edible brown seaweed, E. cava on rotenone-induced neurotoxicity and α-synuclein aggregation in human dopaminergic neuronal cells.
2. Materials and methods
2.1. Preparation of dieckol from Ecklonia cava
The brown seaweed, Ecklonia cava was collected along the coast of Jeju Island, Korea, between October 2014 and March 2015. The samples were washed three times with tap water to remove the salt, epiphytes, and sand attached to the surface, followed by careful rinsing with fresh water, and maintained in a medical refrigerator at −20 °C. Thereafter, the frozen samples were lyophilized and homogenized using a grinder prior to extraction. The phlorotannins were isolated as previously described.18,322.2. Cell culture
A human dopaminergic neuronal cell line, SH-SY5Y cells, were obtained from American Type Culture Collection (ATCC, Manassas, VA). The cells were cultured in in DMEM supplemented with 10% FBS, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, and were maintained in a humidified incubator with 5% CO2. In all experiments, cells were incubated in the presence of the indicated concentrations of dieckol prior to the addition of rotenone. SH-SY5Y cells overexpressing α-synuclein-EGFP were generated via infection with lentiviral vector containing α-synuclein-EGFP cDNA and selection of stably-expressing cells by treatment with puromycin for 2 weeks without colony selection.2.3. Assessment of cell viability
Cell viability was estimated first via an MTT reduction assay.33 For the MTT reduction assay, SH-SY5Y cells (1 × 104 cells per ml) were seeded onto 96-well plates. After 16 h, the cells were pre-treated with vehicle (control) or 5 μM dieckol for 1 h, and subsequently incubated with/without 2 μM rotenone (Sigma, St. Louis, MO, USA) for 24 h at 37 °C. An MTT stock solution (5 mg ml−1) was then added to the wells, to a total reaction volume of 200 μl. After 4 h of the incubation, the plates were centrifuged for 5 min at 800 × g, and the supernatants were aspirated. The formazan crystals in each well were dissolved in 150 μl dimethylsulfoxide (DMSO), and the absorbance was measured at a wavelength of 540 nm. Relative cell viability was evaluated in accordance with the quantity of MTT converted to the insoluble formazan salt. The optical density of the formazan generated in the control cells was considered to represent 100% viability.2.4. Estimation of the intracellular ROS level
SH-SY5Y cells (1 × 105 cells per ml) were seeded onto 6-well plates. The cells were treated with vehicle (control) or 5 μM dieckol, and 1 h later, 500 nM rotenone was added and the cells were incubated for 12 h. Intracellular ROS production was detected by means of an oxidation-sensitive fluorescent probe dye, 2,7-dichlorofluorescein diacetate (DCFH-DA). DCFH-DA was deacetylated intracellularly by nonspecific esterase, which was further oxidized to the highly fluorescent compound dichlorofluorescein (DCF) in the presence of cellular peroxides.34 After changing the media, 5 μg ml−1 DCFH-DA (Invitrogen, San Dieogo, CA) was added and incubated for 30 min at 37 °C. DCF fluorescence intensity was detected by flow cytometry (FACSAriaIII, BD Biosciences). Data were analyzed with BD FACSDiva 7.0 software installed in FACSAriaIII and were presented with WinMDI2.9 software (The Scripps Research Institute, La Jolla, USA).2.5. Western blotting
SH-SY5Y cells were incubated with vehicle (control) or 5 μM dieckol for 1 h and then further incubated with/without 1 μM rotenone for 6 h. The cells were lysed using lysis buffer containing 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.25% sodium deoxycholate, 1% Triton X-100, and protease inhibitor cocktail (GenDEPOT, Barker, TX) for 20 min on ice. The lysates were cleared by centrifugation at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 15 min at 4 °C. The supernatants were mixed with 5× sample buffer, resolved by SDS-PAGE, transferred to PVDF membrane (Millipore, Billerica, MA), and analyzed by immunoblotting using the indicated antibodies. ImageJ (https://imagej.nih.gov/ij/) was used to quantify the band intensity of Western blot.
000 × g for 15 min at 4 °C. The supernatants were mixed with 5× sample buffer, resolved by SDS-PAGE, transferred to PVDF membrane (Millipore, Billerica, MA), and analyzed by immunoblotting using the indicated antibodies. ImageJ (https://imagej.nih.gov/ij/) was used to quantify the band intensity of Western blot.
2.6. Confocal microscopy
EGFP-tagged α-synucelin-overexpressing SH-SY5Y cells cultured on coverslips were incubated with vehicle (control) or 5 μM dieckol for 1 h, after which 250 nM rotenone was added (Sigma-Aldrich, St. Louis, MO, USA). Forty eighth later, the coverslips were washed twice with PBS and fixed in 4% paraformaldehyde for 15 min at room temperature. The fixed cells were then washed with PBS and incubated with PBS containing 0.1% Triton X-100 for 5 min at room temperature. After washing several times with PBS, cells were blocked with PBS containing 1% bovine serum albumin (BSA) and 0.1% Triton X-100 for 30 min at room temperature, and subsequently mounted with vectashield (Vector Laboratories, Burlingame, CA), and observed with a confocal microscope (Zeiss, Germany). To evaluate α-synuclein aggregation, five random fields were selected and cells containing α-synuclein-EGFP puncta were counted by two independent observers blinded to the experimental conditions regardless of cell brightness.2.7. Statistical analysis
All measurements were carried out in triplicate and all values are represented as the mean ± S.E. The results were subjected to an analysis of variance (ANOVA) with the Tukey test to analyze the differences. Values of p < 0.05 were considered significant.3. Results
3.1. Dieckol is prepared from Ecklonia cava
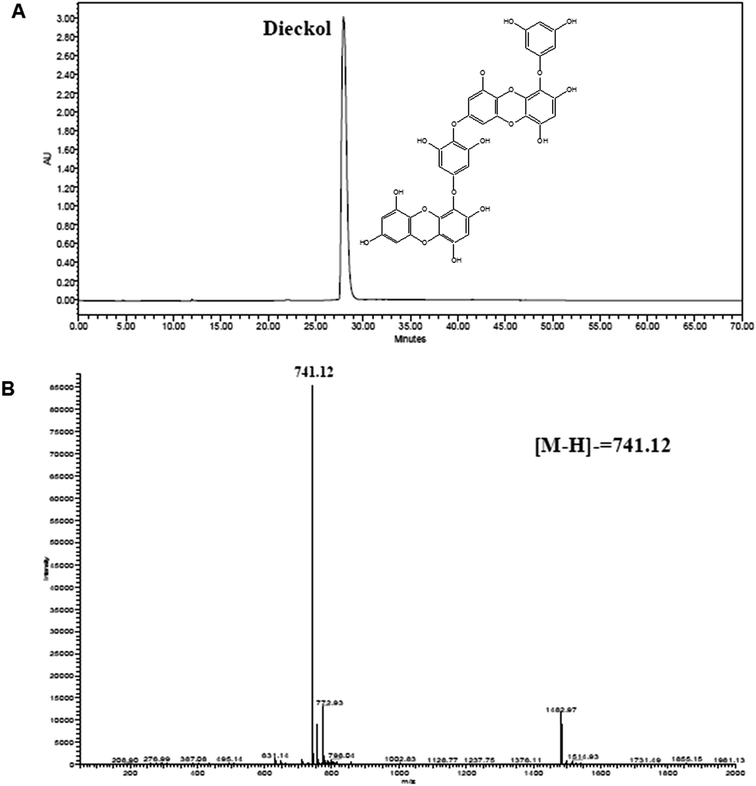
The diethyl ether fraction of E. cava crude extract was subjected to silica gel and Sephadex LH-20 column chromatography and dieckol was purified by reversed-phase HPLC. This purified dieckol was subjected to evaluation of the study. The chemical structure, HPLC chromatogram and HPLC-ESI/MS spectrum of dieckol are presented in Fig. 1. The purity of dieckol was >97%, based on the peak area of component absorbed at specific wavelength in HPLC analysis.3.2. Dieckol attenuates rotenone-induced cytotoxicity in human dopaminergic neuronal cells
In order to determine whether dieckol has a protective effect on rotenone-induced cytotoxicity in human dopaminergic neuronal cells, we treated SH-SY5Y cells, a human dopaminergic neuronal cell line, with rotenone. A significantly lower cell viability was observed in SH-SY5Y cells treated with rotenone (approximately 35% viability), whereas pretreatment with dieckol raised the cell viability to approximately 80–90% in both MTT assay (Fig. 2A) and trypan blue exclusion assay (ESI Fig. 1†). Dieckol alone did not show any cytotoxicity in SH-SY5Y cells at higher concentrations (50 μM) (Fig. 2B), indicating that it possesses a cytoprotective effect against rotenone-induced damage in human dopaminergic neuronal cells.3.3. Dieckol reduces rotenone-induced ROS generation in human dopaminergic neuronal cells
Phlorotannins have been shown to be excellent radical scavengers,18 and dieckol is one of such compound. Therefore, we evaluated whether dieckol scavenges rotenone-induced ROS induction in SH-SY5Y cells. A significantly higher ROS level was observed in SH-SY5Y cells treated with rotenone, whereas ROS production was inhibited by pretreatment with dieckol (Fig. 3). There was no ROS induction following the treatment with dieckol alone, suggesting that dieckol possesses radical scavenging properties in SH-SY5Y cells.3.4. Dieckol reduces rotenone-induced cytochrome C release in human dopaminergic neuronal cells
Rotenone has been known to induce apoptotic cell death by producing ROS.9 Therefore, we evaluated whether dieckol attenuates rotenone-induced cytochrome C release in SH-SY5Y cells. As expected, a higher level of cytochrome C was released upon rotenone treatment, whereas pretreatment with dieckol inhibited this cytochrome C release by rotenone treatment (Fig. 4), suggesting that dieckol possesses mitochondrial protective properties in rotenone-treated SH-SY5Y cells.3.5. Dieckol prevents rotenone-induced α-synuclein aggregation in α-synuclein-overexpressing human dopaminergic neuronal cells
Rotenone has been shown to induce α-synuclein aggregation,35 a major component of Lewy bodies. Therefore, we evaluated whether dieckol retards α-synuclein aggregation. We used EGFP-tagged α-synuclein-overexpressing SH-SY5Y cells. A significantly increased number of intracellular aggregates were observed in rotenone-treated cells (71.4%). Interestingly, pretreatment with dieckol retarded rotenone-induced α-synuclein aggregation (Fig. 5), suggesting that dieckol inhibits rotenone-induced α-synuclein aggregation.4. Discussion
In the present study, we provide compelling evidence of dieckol as a potential therapeutic drug or supplement for PD by showing protective effects of dieckol on typical pathological features of PD; selective dopaminergic neuronal loss and intracellular inclusion body formation, mainly composed of α-synuclein.Firstly, we demonstrate that dieckol prevents dopaminergic neuronal injury from oxidative stress by scavenging free radicals. Dopaminergic neurons are particularly vulnerable to oxidative stress due to the presence of ROS-generating enzymes such as tyrosine hydroxylase.36 In addition, dopaminergic neurons contain high level of iron, which is catalyzed by the Fenton reaction, producing superoxide radicals and hydrogen peroxide, further contributing to oxidative stress. The level of iron has been reported to be increased in the substantia nigra of PD patients.37 Moreover, a decrease in the thiol-reducing agent, glutathione (GSH), has also been observed in PD,38 implying that reduced antioxidant potential may be involved in the oxidative stress related to PD. Although most cells have mechanisms to protect themselves against excessive oxidative stress, unwanted byproducts overwhelm the natural antioxidative defense system. Thus, dietary supplements containing antioxidants may be important for additional protection against oxidative stress and the prevention of neurodegenerative diseases.39,40
In addition to the free radical scavenging activity, other antioxidant mechanisms of dieckol have been reported. Dieckol increases the levels of antioxidant enzymes, including superoxide dismutase, catalase, and glutathione peroxidase,41 and reduces the levels of pro-inflammatory enzymes, including nitric oxide synthase and cyclooxygenase-2 (COX-2),42 thus inhibiting ROS formation. Several signaling pathways induced by excessive ROS have also been identified to be blocked by dieckol; these include ROS-induced apoptotic pathways, the mitochondrial apoptotic pathway, the c-Jun N-terminal kinase (JNK) pathway, and pathways involving NF-kB, caspase-3, and Bax.42–45 The levels of anti-apoptotic mediators including c-IAPs, Bcl-xL, and PARP are increased by dieckol.42,46 Our study also showed that dieckol inhibits rotenone-induced cytochrome C release. Accordingly, dieckol may inhibit ROS-related dopaminergic neuronal damage in three ways: by scavenging ROS, by inhibiting ROS formation, or by blocking ROS-induced apoptotic pathways.
Secondly, we demonstrate that dieckol prevents α-synuclein aggregation induced by rotenone. α-Synuclein aggregates are observed in most PD patients, and they are considered to be a major factor in the pathogenesis of PD.47,48 Therefore, clearance of protein aggregates or retardation of aggregate formation is extremely important in treating PD. Our results evidence that the pretreatment of dieckol significantly decreases rotenone-induced α-synuclein aggregation. It may be due to the antioxidant effect of dieckol. Interestingly, many natural phenolic compounds such as curcumin, epigallocatechin-gallate (EGCG), and resveratrol, show inhibition of amyloid protein aggregation, and the molecular mechanisms underlying this action are stabilization of native states or remodeling and inactivation of toxic amyloid oligomers in addition to antioxidant effects.39 When added to preformed fibrils, curcumin, a diphenol derived from the rhizome of Curcuma longa, increases the solubility of α-synuclein monomers, thus dose-dependently preventing protein oligomerization and reducing the amount of high molecular weight aggregates.49 EGCG, which is a phenolic constituent originating from green tea, has also been reported to remodel mature α-synuclein fibrils and reduce cellular toxicity.2,50 Accordingly, dieckol may reduce α-synuclein aggregation via different mechanisms in addition to antioxidant effects.
Dieckol has been reported to cross the BBB,51 implying that it is advantageous as a candidate for direct neuroprotective and neuromodulatory actions. Moreover, the majority of PD patients have concomitant sleep disturbances and depression.52–55 Interestingly, phlorotannins of E. cava improve not only depression but also sleep disturbance by modulating the benzodiazepine binding site of the gamma-aminobutyric acid type A (GABAA) receptor,31,56 suggesting that dieckol can help patients with PD in different ways.
In conclusion, the results obtained in the present study shows that dieckol isolated from E. cava can effectively inhibits intracellular ROS formation and human dopaminergic neuronal cytotoxicity induced by rotenone. Moreover, dieckol has protective effects on mitochondrial function and prevention of α-synuclein aggregation. These results reveal that dieckol can be used as an ingredient for functional food and pharmaceutical agents related to PD.
Conflict of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This work was supported by the MRC Program (2012R1A5A2048183) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (2014R1A1A3050501; 2015R1A2A2A01007457).References
- A. J. Lees, J. Hardy and T. Revesz, Lancet, 2009, 373, 2055–2066 CrossRef CAS.
- Y. Xu, Y. Zhang, Z. Quan, W. Wong, J. Guo, R. Zhang, Q. Yang, R. Dai, P. L. McGeer and H. Qing, Neurochem. Res., 2016, 41, 2788–2796 CrossRef CAS PubMed.
- K. F. Winklhofer and C. Haass, Biochim. Biophys. Acta, Gen. Subj., 2010, 1802, 29–44 CrossRef CAS PubMed.
- O. Hwang, Experimental Neurobiology, 2013, 22, 11–17 CrossRef PubMed.
- V. Dias, E. Junn and M. M. Mouradian, J. Parkinson's Dis., 2013, 3, 461–491 CAS.
- C. M. Tanner, F. Kamel, G. W. Ross, J. A. Hoppin, S. M. Goldman, M. Korell, C. Marras, G. S. Bhudhikanok, M. Kasten, A. R. Chade, K. Comyns, M. B. Richards, C. Meng, B. Priestley, H. H. Fernandez, F. Cambi, D. M. Umbach, A. Blair, D. P. Sandler and J. W. Langston, Environ. Health Perspect., 2011, 119, 866–872 CrossRef CAS PubMed.
- C. Freire and S. Koifman, NeuroToxicology, 2012, 33, 947–971 CrossRef CAS PubMed.
- F. D. Dick, G. De Palma, A. Ahmadi, N. W. Scott, G. J. Prescott, J. Bennett, S. Semple, S. Dick, C. Counsell, P. Mozzoni, N. Haites, S. B. Wettinger, A. Mutti, M. Otelea, A. Seaton, P. Soderkvist and A. Felice, Occup. Environ. Med., 2007, 64, 666–672 CrossRef CAS PubMed.
- N. Li, K. Ragheb, G. Lawler, J. Sturgis, B. Rajwa, J. A. Melendez and J. P. Robinson, J. Biol. Chem., 2003, 278, 8516–8525 CrossRef CAS PubMed.
- P. T. Bywood and S. M. Johnson, Exp. Neurol., 2003, 179, 47–59 CrossRef CAS PubMed.
- W. Xiang, J. C. Schlachetzki, S. Helling, J. C. Bussmann, M. Berlinghof, T. E. Schaffer, K. Marcus, J. Winkler, J. Klucken and C. M. Becker, Mol. Cell. Neurosci., 2013, 54, 71–83 CrossRef CAS PubMed.
- S. C. Ko, M. Lee, J. H. Lee, S. H. Lee, Y. Lim and Y. J. Jeon, Environ. Toxicol. Pharmacol., 2013, 36, 1253–1260 CrossRef CAS PubMed.
- J. R. Cannon, V. Tapias, H. M. Na, A. S. Honick, R. E. Drolet and J. T. Greenamyre, Neurobiol. Dis., 2009, 34, 279–290 CrossRef CAS PubMed.
- H. Rodriguez-Rocha, A. Garcia-Garcia, C. Pickett, S. Li, J. Jones, H. Chen, B. Webb, J. Choi, Y. Zhou, M. C. Zimmerman and R. Franco, Free Radical Biol. Med., 2013, 61, 370–383 CrossRef CAS PubMed.
- N. Xiong, X. Long, J. Xiong, M. Jia, C. Chen, J. Huang, D. Ghoorah, X. Kong, Z. Lin and T. Wang, Crit. Rev. Toxicol., 2012, 42, 613–632 CrossRef CAS PubMed.
- T. Kuda, M. Tsunekawa, H. Goto and Y. Araki, J. Food Compos. Anal., 2005, 18, 625–633 CrossRef CAS.
- Y. Athukorala and Y.-J. Jeon, J. Food Sci. Nutr., 2005, 10, 134–139 Search PubMed.
- G.-N. Ahn, K.-N. Kim, S.-H. Cha, C.-B. Song, J. Lee, M.-S. Heo, I.-K. Yeo, N.-H. Lee, Y.-H. Jee and J.-S. Kim, Eur. Food Res. Technol., 2007, 226, 71–79 CrossRef CAS.
- T. Shibata, K. Ishimaru, S. Kawaguchi, H. Yoshikawa and Y. Hama, J. Appl. Phycol., 2008, 20, 705–711 CrossRef CAS.
- K. Kang, J. H. Hye, H. H. Dong, Y. Park, H. K. Seong, H. L. Bong and H. C. Shin, Res. Commun. Mol. Pathol. Pharmacol., 2004, 115–116, 77–95 CAS.
- K. Nagayama, Y. Iwamura, T. Shibata, I. Hirayama and T. Nakamura, J. Antimicrob. Chemother., 2002, 50, 889–893 CrossRef CAS PubMed.
- S. J. Heo, E. J. Park, K. W. Lee and Y. J. Jeon, Bioresour. Technol., 2005, 96, 1613–1623 CrossRef CAS PubMed.
- K. N. Kim, S. J. Heo, C. B. Song, J. Lee, M. S. Heo, I. K. Yeo, K. A. Kang, J. W. Hyun and Y. J. Jeon, Process Biochem., 2006, 41, 2393–2401 CrossRef CAS.
- S. J. Heo, S. C. Ko, S. H. Cha, D. H. Kang, H. S. Park, Y. U. Choi, D. Kim, W. K. Jung and Y. J. Jeon, Toxicol. in Vitro, 2009, 23, 1123–1130 CrossRef CAS PubMed.
- M. C. Kang, S. H. Cha, W. A. Wijesinghe, S. M. Kang, S. H. Lee, E. A. Kim, C. B. Song and Y. J. Jeon, Food Chem., 2013, 138, 950–955 CrossRef CAS PubMed.
- S. J. Heo, S. H. Cha, K. N. Kim, S. H. Lee, G. Ahn, D. H. Kang, C. Oh, Y. U. Choi, A. Affan, D. Kim and Y. J. Jeon, Appl. Biochem. Biotechnol., 2012, 166, 1520–1532 CrossRef CAS PubMed.
- H. A. Jung, S. E. Jin, B. R. Ahn, C. M. Lee and J. S. Choi, Food Chem. Toxicol., 2013, 59, 199–206 CrossRef CAS PubMed.
- S. Y. Shim, L. Quang-To, S. H. Lee and S. K. Kim, Food Chem. Toxicol., 2009, 47, 555–560 CrossRef CAS PubMed.
- Na Y. Yoon, H. Y. Chung, H. R. Kim and A. J. S. Cho, Fish. Sci., 2008, 74, 200–207 CrossRef CAS.
- C. S. Myung, H. C. Shin, H. Y. Bao, S. J. Yeo, B. H. Lee and J. S. Kang, Arch. Pharmacal Res., 2005, 28, 691–698 CrossRef CAS.
- H. Y. Suengmok Cho, Y.-J. Jeon, C. Justin Lee, Y.-H. Jin, N.-I. Baek, S.-M. K. Dongsoo Kim, M. Yoon, H. Yong, M. Shimizu and D. Han, Food Chem., 2012, 132 Search PubMed.
- E.-A. Kim, S.-H. Lee, J.-H. Lee, N. Kang, J.-Y. Oh, S.-h. Cha, G. Ahn, S.-C. Ko, I. P. S. F. Fernando, S.-Y. Kim, S. J. Park, Y.-T. Kim and Y.-J. Jeon, RSC Adv., 2016, 6, 78570 RSC.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
- A. R. Rosenkranz, S. Schmaldienst, K. M. Stuhlmeier, W. Chen, W. Knapp and G. J. Zlabinger, J. Immunol. Methods, 1992, 156, 39–45 CrossRef CAS PubMed.
- S. Dadakhujaev, H. S. Noh, E. J. Jung, J. Y. Cha, S. M. Baek, J. H. Ha and D. R. Kim, Neurosci. Lett., 2010, 472, 47–52 CrossRef CAS PubMed.
- J. Haavik, B. Almas and T. Flatmark, J. Neurochem., 1997, 68, 328–332 CrossRef CAS PubMed.
- H. Mochizuki and T. Yasuda, J. Neural Transm., 2012, 119, 1511–1514 CrossRef CAS PubMed.
- J. Sian, D. T. Dexter, A. J. Lees, S. Daniel, Y. Agid, F. Javoy-Agid, P. Jenner and C. D. Marsden, Ann. Neurol., 1994, 36, 348–355 CrossRef CAS PubMed.
- M. Stefani and S. Rigacci, Int. J. Mol. Sci., 2013, 14, 12411–12457 CrossRef PubMed.
- M. Solayman, M. Asiful Islam, F. Alam, M. I. Khalil, M. A. Kamal and S. H. Gan, Curr. Drug Metab., 2016 DOI:10.2174/1389200217666160709204826.
- M.-C. Kang, S.-M. Kang, G. Ahn, K.-N. Kim, N. Kang, K. W. Samarakoon, M.-C. Oh, J.-S. Lee and Y.-J. Jeon, Environ. Toxicol. Pharmacol., 2013, 35, 517–523 CrossRef CAS PubMed.
- Y. I. Yang, J. H. Woo, Y. J. Seo, K. T. Lee, Y. Lim and J. H. Choi, J. Agric. Food Chem., 2016, 64, 570–578 CrossRef CAS PubMed.
- J. H. Ahn, Y. I. Yang, K. T. Lee and J. H. Choi, J. Cancer Res. Clin. Oncol., 2015, 141, 255–268 CrossRef CAS PubMed.
- Y. J. Jeon, H. S. Kim, K. S. Song, H. J. Han, S. H. Park, W. Chang and M. Y. Lee, Drug Chem. Toxicol., 2015, 38, 180–187 CrossRef CAS PubMed.
- S. Y. Lee, J. Lee, H. Lee, B. Kim, J. Lew, N. Baek and S. H. Kim, J. Agric. Food Chem., 2016, 64, 5508–5514 CrossRef CAS PubMed.
- S. H. Lee, J. Y. Kim, S. Y. Yoo and S. M. Kwon, Free Radical Res., 2013, 47, 526–534 CrossRef CAS PubMed.
- L. Stefanis, Cold Spring Harbor Perspect. Med., 2012, 2, a009399 Search PubMed.
- S. Gandhi and A. Y. Abramov, Oxid. Med. Cell. Longevity, 2012, 2012, 428010 Search PubMed.
- N. Pandey, J. Strider, W. C. Nolan, S. X. Yan and J. E. Galvin, Acta Neuropathol., 2008, 115, 479–489 CrossRef CAS PubMed.
- J. Bieschke, J. Russ, R. P. Friedrich, D. E. Ehrnhoefer, H. Wobst, K. Neugebauer and E. E. Wanker, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 7710–7715 CrossRef CAS PubMed.
- J. H. Kwak, Z. Yang, B. Yoon, Y. He, S. Uhm, H.-C. Shin, B. H. Lee, Y. C. Yoo, K. B. Lee, S.-Y. Han and J. S. Kim, Biomaterials, 2015, 61, 52–60 CrossRef CAS PubMed.
- B. Faludi, J. Janszky, S. Komoly and N. Kovacs, Orv. Hetil., 2015, 156, 1091–1099 CrossRef PubMed.
- K. Suzuki, M. Miyamoto, T. Miyamoto, M. Iwanami and K. Hirata, Parkinson's Dis., 2011, 2011, 219056 Search PubMed.
- K. A. Bjornara, E. Dietrichs and M. Toft, Clin. Neurol. Neurosurg., 2014, 124, 37–43 CrossRef PubMed.
- H. Rickards, J. Neurol., Neurosurg. Psychiatry, 2005, 76(1), i48–52 CrossRef PubMed.
- S. Cho, D. Han, S.-B. Kim, M. Yoon, H. Yang, Y.-H. Jin, J. Jo, H. Yong, S.-H. Lee, Y.-J. Jeon and M. Shimizu, Biosci., Biotechnol., Biochem., 2012, 76, 163–168 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21697h |
| This journal is © The Royal Society of Chemistry 2016 |