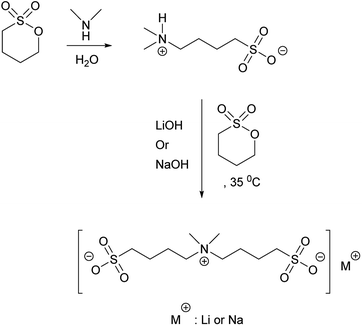
Fluorine-free salts for aqueous lithium-ion and sodium-ion battery electrolytes†
Elham
Hosseini-Bab-Anari‡
 a,
Andrea
Boschin‡
b,
Toshihiko
Mandai
b,
Hyuma
Masu
c,
Kasper
Moth-Poulsen
a,
Andrea
Boschin‡
b,
Toshihiko
Mandai
b,
Hyuma
Masu
c,
Kasper
Moth-Poulsen
 *a and
Patrik
Johansson
*a and
Patrik
Johansson
 *b
*b
aDepartment of Chemistry and Chemical Engineering, Chalmers University of Technology, 412 96 Gothenburg, Sweden. E-mail: kasper.moth-poulsen@chalmers.se
bDepartment of Physics, Chalmers University of Technology, 412 96 Gothenburg, Sweden. E-mail: patrik.johansson@chalmers.se
cCenter for Analytical Instrumentation, Chiba University, 1-33 Yayoi-cho, Inage-ku, Chiba, 263-8522, Japan
First published on 30th August 2016
Abstract
A first generation of fluorine-free lithium and sodium salts based on the concept of pseudo-delocalized anions has been synthesized with both high purity and yield using water as the solvent in the reaction medium. The salts have been fully characterized by Raman and FT-IR spectroscopies, thermogravimetry, and X-ray crystallography to reveal both basic properties in terms of thermal stability and solubility as well as the local, mainly ion–ion interaction dictated, coordination details and by ionic conductivity and electrochemical stability window measurements as aqueous electrolytes. Together a picture is created of the salts' promise as components in electrolytes – primarily aiming at application in low voltage fluorine-free aqueous lithium-ion batteries (LIBs) and sodium-ion batteries (SIBs).
1. Introduction
The increasing usage of lithium-ion batteries (LIBs) in rechargeable personal mobile devices such as cell phones, cameras, laptops, and more recently also in electric vehicles (EV), will eventually lead to an accumulation of millions of tonnes of non-environmentally-friendly waste material.1 Each LIB cell is composed of three main components, the two electrodes and the electrolyte, together with peripherals such as current collectors, casing, etc. While several research reports focus on the making and use of eco-friendly electrodes2–6 there are much fewer cases of environmentally friendly electrolytes, which perhaps are the more problematic parts to recycle and amount to ca. 5–10% of the total cell weight. The current commercially used electrolytes all contain fluorinated lithium salts, typically based on the meta-stable PF6− anion, which can decompose upon reaction with traces of water and release toxic gases such as HF and POF3 in case of critical conditions or abuse.7–14 This thus apart from the fluorine synthesis itself being non-environmentally friendly, dangerous15 and costly.16 The electrolytes also contain highly flammable and often environmentally demanding, sometimes toxic, solvents, typically a mixture of ethylene carbonate and dimethyl carbonate (EC/DMC). To avoid any unwanted reactions, moisture-free production and/or using special additives is vital, and in the end this drastically increases the cost of the LIBs.17–19However, some safer weakly coordinating anions (WCAs), but still fluorinated, have been synthesized such as FSI (FSO2NSO2F), TFSI (CF3SO2NSO2CF3), and “Hückel-anions” like 2-trifluoromethyl-4,5-dicyanoimidazole (TDI), all with covalent bonds to the F atoms.20,21 However, as these WCAs still contain fluorine, some of the safety concerns still remain. Even more attractive alternatives are totally fluorine-free WCAs,22,23 such as TADC24,25 and PCPI26 and/or aqueous electrolyte based technologies.27–31
Another WCA example was reported in 2012 by Jónsson et al.,32,33 with their design of fluorine-free pseudo-delocalized anions, also known as “Mickey Mouse™” (MM) anions (Fig. 1a). Here, a positively charged unit is flanked by two covalently attached negatively charged groups – none of them fluorinated. These anions have a partially delocalized charge, as the charge is neither completely localized to a unique region nor delocalized as for most other WCAs, why the term pseudo-delocalized seems appropriate.
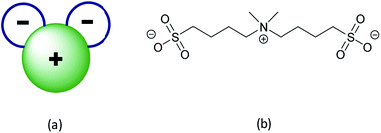 | ||
| Fig. 1 (a) The concept of pseudo-delocalized “Mickey Mouse™” anions and (b) (di-methyl ammonio)bis(butane-1-sulfonate), MM4411. | ||
Despite the attractiveness of the original design of MM anions, to date only a few of the proposed structures has been prepared34 and none has been fully characterized as lithium or sodium salts in the context of future battery application. Here we have developed a synthesis strategy for the fluorine-free aliphatic anion 4,4′-(di methyl ammonio)bis(butane-1-sulfonate) (MM4411, Fig. 1b) and its lithium and sodium salts, LiMM4411 and NaMM4411, respectively (Scheme 1).
2. Experimental
2.1. Materials
All chemicals were used as received. Di-methyl amine solution 40 wt% in H2O, 1,4-butane sultone, lithium hydroxide powder (≥98%), sodium hydroxide pellet (≥97%), ethylene carbonate (EC; anhydrous 99.8%), propylene carbonate (PC; anhydrous 99.7%), di-methyl carbonate (DMC; anhydrous, 99%), and acetonitrile (AN; 99.8%) were all purchased from Sigma-Aldrich. Ultrapure water was obtained from a Direct-Q® (Merck Millipore) purification system. Poly(ethylene oxide), (PEO), Mw: 5 × 106 g mol−1 was obtained from Polysciences (4031). Platinum foil, 99.95%, was obtained from Advent Research Materials Ltd. and glass microfiber separators were obtained from Whatman.2.2. Analytical methods
Both the Li and Na salt were characterized by NMR spectroscopy, LC-MS and elemental analysis. 1H NMR and 13C NMR spectra were recorded using a Bruker 400 MHz spectrometer at 298 K, using the solvent residual peak as internal standard for H in D2O. The elemental analysis was done as a service by Mikrolab Kolbe AG, Germany. X-ray crystallography was performed on a Bruker Smart Apex II diffractometer equipped with a CCD area detector using monochromatic Mo Kα radiation (λ = 0.71073 Å). Thermal analysis was performed by thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC) using a TG 209 F1 Iris from Netzsch and a DSC Q1000 from TA instrument, respectively. The Raman spectra were recorded using a Bruker MultiRAM and the FTIR spectroscopy was carried out on Bruker Alpha equipped with an ATR module. Ionic conductivity measurements were performed using a Novocontrol broadband dielectric spectrometer and a nitrogen gas cryostat (Quatro cryosystem) was used to control the temperature of the cell. The electrochemical stability window (ESW) was evaluated with an IVIUM n-stat (IVIUM Technologies B.V.).2.3. Synthesis procedures
Zwitterionic di-methyl ammonio-butane sulfonate (ZDMBS) was obtained as a colorless crystalline crude product. The crude was recrystallized from ethanol (96%). Upon drying in a vacuum oven at 50 °C for 24 h, the target compound, 10 g, i.e. 55 mmol, corresponding to a 94% yield, was achieved as analytically pure colorless crystals.
ZDMBS. 1H-NMR (400 MHZ, D2O): 3.05 (t, 2H, NCH2); 2.83 (t, 2H, SCH2); 2.75 (s, 6H, 2 × CH3); 1.62–1.80 (m, 4H, –CH2 and–CH2–). 13C-NMR (100 MHz, D2O): 57.1 (NCH2); 49.9 (SCH2); 42.6 (2 × CH3); 22.8 (CH2); 21.1 (CH2). Elemental analysis calcd for C6H15NO3: C, 39.76; H, 8.34; N, 7.73; S, 17.69; O, 26.5. Found: C, 40.02; H, 8.54; N, 7.97; S, 17.2; O, 25.9.
LiMM4411. 1H-NMR (400 MHZ, D2O): 3.21 (t, 4H, NCH2); 2.93 (s, 6H, 2 × CH3); 2.84 (t, 4H, SCH2); 1.8 (m, 4H, –CH2); 1.67 (m, 4H, –CH2–). 13C-NMR (100 MHZ, D2O): 63.5 (NCH2); 50.4 (SCH2); 49.9 (2 × CH3); 21 (CH2); 20.7 (CH2). Elemental analysis calcd for LiC10H22NO6S2: C, 37.15; H, 6.86; N, 4.33; S, 19.83; Li, 2.15. Found: C, 36.83; H, 6.69; N, 4.21; S, 18.93; Li, 2.07.
NaMM4411. 1H-NMR (400 MHZ, D2O): 3.2 (t, 4H, NCH2); 2.93 (s, 6H, 2 × CH3); 2.83 (t, 4H, SCH2); 1.79 (m, 4H, –CH2); 1.66 (m, 4H, –CH2–). 13C-NMR (100 MHZ, D2O): 63.5 (NCH2); 50.4 (SCH2); 49.9 (2 × CH3); 21 (CH2); 20.7 (CH2). Elemental analysis calcd for NaC10H22NO6S2: C, 35.39; H, 6.53; N, 4.13; S, 18.89; Na, 6.77. Found: C, 35.78; H, 6.76; N, 3.99; S, 18.00; Na, 6.47. All the NMR spectra are available in the ESI.†
2.4. Crystallography
Single crystals of LiMM4411 and NaMM4411 suitable for X-ray crystallography were obtained by recrystallization from concentrated aqueous solutions. A single crystal was mounted on a glass pin with a minimal amount of epoxy resin and cooled to −100 °C using a steady flow of nitrogen gas stream. An empirical absorption correction was applied using a multi-scan averaging of symmetry equivalent data in the SADABS program.36 The structures were solved by the direct method using SHELXS-97 and refined as full-matrix least-squares anisotropically for non-hydrogen atoms with SHELXL-2013.37,38 All hydrogen atoms, excepting for those on water molecules, were placed at their geometrically ideal positions (methyl H atoms allowed to rotate but not to tip) and refined using the riding model. The remaining water hydrogen atoms for LiMM4411 were determined from the corresponding Fourier peak, while the positions of those for NaMM4411 were unclear due to disorder. The crystallographic information file (cif) was deposited in the Cambridge Structure Database (CSD) as CCDC 1492164 and 1492165 for LiMM4411 and NaMM4411, respectively.Crystal data for [LiMM4411·(H2O)2]·H2O: C10H22LiNO6S2·3H2O, Mw = 377.40, monoclinic, space group Cc (no. 9), a = 14.4370(12), b = 15.0453(12), c = 9.4300(8) Å, β = 122.2871(9), V = 1731.6(2) Å3, Z = 4, Dcalc = 1.448 g cm−3, μ = 0.349 mm−1, T = 173 K, 4912 total reflections, 2432 unique reflections, Rint = 0.0274, R1(I > 2σ(I)) = 0.0302, R1(all data) = 0.0354, wR2(I > 2σ(I)) = 0.0642, wR2(all data) = 0.0672, GooF = 1.072, residual minimum and maximum electron densities −0.305 and 0.298 e Å−3, respectively.
Crystal data for [NaMM4411·H2O]: C10H22NNaO6S2·H2O, Mw = 355.39, monoclinic, space group C2/c (no. 15), a = 29.6781(16), b = 10.9086(6), c = 9.8067(5) Å, β = 104.1423(6), V = 3078.7(3) Å3, Z = 8, Dcalc = 1.534 g cm−3, μ = 0.404 mm−1, T = 173 K, 8673 total reflections, 2453 unique reflections, Rint = 0.0220, R1(I > 2σ(I)) = 0.0359, R1(all data) = 0.0462, wR2(I > 2σ(I)) = 0.1228, wR2(all data) = 0.1398, GooF = 1.051, residual minimum and maximum electron densities −0.379 and 0.563 e Å−3, respectively.
2.5. Solubility tests
Solubility tests were performed by adding liquid solvents; PC, EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1
DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, ACN, and water, to the LiMM4411 and NaMM4411 salts and subsequently mixing with a magnetic stirrer. The solubility was evaluated by simple visual inspection. In addition, solid polymer electrolytes (SPEs) with the compositions LiMM4411(PEO)20 and NaMM4411(PEO)20, respectively, were made by using appropriate amounts of salt and PEO and water as a secondary solvent. After stirring the solutions for one day, casting was made in a PTFE mould. After evaporation of most of the water, the casted films were further dried in a Büchi vacuum oven (<7 Pa) at 50 °C for 24 h, in order to eliminate any residual water. The resulting SPEs were evaluated in terms of salt solvation using Raman spectroscopy and DSC.
1, ACN, and water, to the LiMM4411 and NaMM4411 salts and subsequently mixing with a magnetic stirrer. The solubility was evaluated by simple visual inspection. In addition, solid polymer electrolytes (SPEs) with the compositions LiMM4411(PEO)20 and NaMM4411(PEO)20, respectively, were made by using appropriate amounts of salt and PEO and water as a secondary solvent. After stirring the solutions for one day, casting was made in a PTFE mould. After evaporation of most of the water, the casted films were further dried in a Büchi vacuum oven (<7 Pa) at 50 °C for 24 h, in order to eliminate any residual water. The resulting SPEs were evaluated in terms of salt solvation using Raman spectroscopy and DSC.
2.6. Thermal analysis
For the TGA measurements an aluminum crucible was loaded with ca. 10 mg of sample and placed in the sample compartment. All samples were heated from 25 °C to 500 °C at a rate of 5 °C min−1 under a dry nitrogen gas flow of 100 mL min−1. The decomposition temperatures (Td) were defined as the temperature of 1% mass loss. DSC measurements were conducted with ca. 10 mg of sample sealed in an aluminum pan inside the glove box. The samples were first cooled from 40 °C to −100 °C and subsequently heated to 280 °C (the SPEs only to 150 °C), whereafter they were cooled down to −100 °C again, all at 10 °C min−1, under a flow of helium gas.2.7. Raman and IR spectroscopy
The FT-Raman spectra were collected using the 1064 nm line of a Nd:YAG laser as excitation source with a power of 250 mW, a liquid nitrogen cooled germanium detector, and at a resolution of 2 cm−1. The Blackmann–Harris 3 term apodization function was used39 and the spectra averaged over 400 scans. The samples were placed in glass vials properly sealed inside the glove box to avoid influence from any moisture.ATR-FTIR spectra were collected in the range 375–4000 cm−1 with a resolution of 2 cm−1 and averaged over 400 scans using a diamond plate as the refractive element – all inside the glove box. The Blackmann–Harris 3 term apodization function was used also here.39
2.8. Ionic conductivity
Ionic conductivity measurements were performed on 1.5 m aqueous solutions of LiMM4411 and NaMM4411, in the range 10−2 to 10−7 Hz. The samples were placed in a cell of a PTFE ring spacer, inserted between two blocking stainless steel electrodes. The spacer had an inner diameter of 13.5 mm and a thickness of 0.95 mm. The cell was heated from 0 °C to 100 °C and thereafter cooled to 0 °C with steps of 10 °C, with an equilibration time of 30 min. The DC conductivity (σ) was evaluated with the following formula:where R is the bulk resistance, as obtained from a Nyquist plot, t and d are the thickness and the inner diameter of the PTFE ring spacer, respectively.
2.9. Electrochemical stability window (ESW)
The ESWs of 1.5 m aqueous solutions of LiMM4411 and NaMM4411 salts were evaluated by performing linear sweep voltammetry (LSV) at room temperature with a three electrode cell. Two platinum discs, 1 cm diameter, served as working and counter electrodes and an Ag/AgCl served as reference electrode. A disc of glass microfiber was used as separator and soaked with the electrolyte. The cathodic scan was performed from open circuit voltage (OCV) to −1.5 V vs. Ag/AgCl, while the anodic scan was performed from OCV to 2 V vs. Ag/AgCl, both at a scan rate of 1 mV s−1.3. Results and discussion
3.1. Synthesis of lithium and sodium salts
As mentioned above both the lithium, LiMM4411, and the sodium, NaMM4411, salts were synthesized using a two-step procedure, from inexpensive and readily available starting materials such as di-methyl amine and butane sultone. The methods reported here were carried out in water and can be favourably compared to a very recently reported synthesis route to the aliphatic anion (HMM4411)34 using somewhat different conditions. In the synthesis route presented here, a monomeric zwitterionic precursor was first obtained by nucleophilic substitution using di-methyl amine as the nucleophile leading to simultaneous ring opening of the butane sultone ring, which is a common reaction for preparation of sulfobetaine compounds.40,41 Using an excess of di-methyl amine solution in the first step was found to be essential, as otherwise a mixture of mono and di-substituted amines was obtained,40 leading to difficulties in isolation of the desired product. An alternative could be to add an extra amount of butane sultone (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) to the di-methyl amine solution, whereby a mix of the desired product in its acidic form plus an extra amount of butane sultone would likely be achived.34 However, lower reaction yield and using excess of butane sultone, made it more attractive to perform the reaction in two steps for higher purity and yield. Reaction step 2 was found to be temperature dependent, as monitored by NMR, and hence increasing the temperature led to a faster completion but at the expense of the conversion. Therefore, in order to optimize yield and purity, the effect of different temperatures; 20, 35, 50, 75, and 100 °C, was studied resulting in the following reaction completion times and conversions; 21 days, 99%; 72 h, 98%; 48 h, 91%; 36 h, 87%, and 24 h, 83%. As the best compromise, 35 °C was selected as the reaction temperature. The origin of the lower completion is that 1,4-butane sultone tends to react with water42 and produce hydroxyl butanesulfonic acid at higher temperatures. Using this approach, the target compound, zwitterionic di-methyl ammonio-butanesulfonate (ZDMBS) could be obtained analytically pure in 94% yield after recrystallization. Reaction of ZDMBS with NaOH or LiOH and butane sultone in water lead to the formation of the lithium and sodium salt of 4,4′-(di-methyl ammonio)bis(butane-1-sulfonate), LiMM4411 and NaMM4411, as analytically pure white crystals in 90 and 94% yield respectively. This optimized synthesis route furnish the target molecules analytically pure in excellent yields with all steps carried out on multiple gram scale using water as the only solvent in reaction media. Since no chromatography steps are involved, we envision that future scale-up of this method to the kg scale will be rather straight-forward.
1 molar ratio) to the di-methyl amine solution, whereby a mix of the desired product in its acidic form plus an extra amount of butane sultone would likely be achived.34 However, lower reaction yield and using excess of butane sultone, made it more attractive to perform the reaction in two steps for higher purity and yield. Reaction step 2 was found to be temperature dependent, as monitored by NMR, and hence increasing the temperature led to a faster completion but at the expense of the conversion. Therefore, in order to optimize yield and purity, the effect of different temperatures; 20, 35, 50, 75, and 100 °C, was studied resulting in the following reaction completion times and conversions; 21 days, 99%; 72 h, 98%; 48 h, 91%; 36 h, 87%, and 24 h, 83%. As the best compromise, 35 °C was selected as the reaction temperature. The origin of the lower completion is that 1,4-butane sultone tends to react with water42 and produce hydroxyl butanesulfonic acid at higher temperatures. Using this approach, the target compound, zwitterionic di-methyl ammonio-butanesulfonate (ZDMBS) could be obtained analytically pure in 94% yield after recrystallization. Reaction of ZDMBS with NaOH or LiOH and butane sultone in water lead to the formation of the lithium and sodium salt of 4,4′-(di-methyl ammonio)bis(butane-1-sulfonate), LiMM4411 and NaMM4411, as analytically pure white crystals in 90 and 94% yield respectively. This optimized synthesis route furnish the target molecules analytically pure in excellent yields with all steps carried out on multiple gram scale using water as the only solvent in reaction media. Since no chromatography steps are involved, we envision that future scale-up of this method to the kg scale will be rather straight-forward.
3.2. Crystallography
The structures of the salts provide some insight into solubility issues and can guide future molecular design. LiMM4411 crystallizes in the monoclinic crystal system with a Cc space group and NaMM4411 in the same crystal system with a C2/c space group. The thermal ellipsoid models of LiMM4411 and NaMM4411 (Fig. 2) show solvent water molecules involved as ligands and/or being co-crystallized, as often found for alkali metal salts,43–46 likely due to the preferential coordination number of each alkali metal ion in the crystalline state and the high compatibility/affinity of the salts with water (vide infra).In the crystal of LiMM4411, the Li+ is surrounded by four oxygen atoms from two different SO3− groups and two different water molecules in a tetrahedral geometry. Another water molecule, mediating the hydrogen-bonding network, is also co-crystallized giving in total three water molecules incorporated per cation–anion pair. The Li–OSO3 and Li–Owater distances are in the range 1.956–1.980 and 1.906–1.938 Å, respectively, indicating comparable Lewis acid–base interactions of the SO3− groups to those of water. On the other hand, in the crystal of NaMM4411 the Na+ is penta-coordinated in a square-pyramidal manner by five oxygen atoms from four different SO3− groups and one water molecule. The Na–OSO3 and Na–Owater distances are here 2.280–2.358 and 2.562 Å, respectively, and thus considerably longer than the Li–O distances in LiMM4411, rather easily understandable from the Lewis acidities (charge densities) of the two alkali metal ions.
The packing structures are also strongly dependent on the alkali metal ion; LiMM4411 forms a 3D architecture where the tetrahedral Li coordination spheres are located as a zig-zag pattern, while there is a 2D hexagonal grid supramolecular network in NaMM4411 (Fig. 3).
 | ||
| Fig. 3 Packing diagrams of (top) LiMM4411 and (down) NaMM4411 along the c-axis. Pink, Li; blue, Na; gray, C; white, H; red, O; light blue, N; yellow, S. | ||
This unexpected large difference probably arises from the conformational flexibility of the butylene moieties, the preferential coordination (combined with steric effects) for each alkali metal ion, and hydrogen bonds (HBs). For the LiMM4411 crystal, the conformation of both butylene moieties is tg′t (Table S1;†t and g mean trans and gauche conformation, respectively), leading to a close proximity of terminal (SO3− and Li+) and centre (nitrogen) moieties.47,48 The tetrahedral coordination geometry likely allows for short contacts to both the bulky SO3− groups and the small water molecules, promoting the formation of a HB network. HBs often play a key role to stabilize and/or to form the characteristic crystal structures.49–53 The three water molecules within the LiMM4411 crystal participate in the HB network as both donors and acceptors (Table S2†). These structural features all contribute cooperatively to form the 3D aggregated crystal structure of the LiMM4411. In contrast, in the NaMM4411 crystal the anion has one tg′t and one ttt conformation butylene unit, resulting in an L-shaped structure. The relatively crowded square–pyramidal geometry around the Na+ combined with the L-shaped framework leads to the ladder like 2D arrangement. The precise positions of water hydrogen atoms are unknown, and hence the HB network in the NaMM4411 crystal is ambiguous, but HBs seem to be less as the water molecule (O7) is not surrounded by any hydrogen atoms (while in the proximity of other oxygen atoms).
3.3. Solubility
The solubility of the salts, listed in Table 1, can from the computational screening study be expected to be quite low as compared to other pseudo-delocalized anion based salts.32 Therefore, and despite the high dielectric constants of PC (63) and EC (90),54 clear solutions are only obtained in a protic solvents as water. This thus confirms the strong interactions of the two SO3− groups to the Li+ or Na+ ions – as also found in the single crystals. Water, as opposed to EC and PC, can act to relax the HB network or extend it to replace the interactions of the SO3− groups with the alkali metal ions. Turning to the SPEs, there are no signs of the typical features assignable to the complexation of PEO with Li+ or Na+ detected neither with Raman spectroscopy nor with DSC (not shown), and thus the LiMM4411 and NaMM4411 salts are not considered solvated by the PEO.55–57 Altogether, these findings open for application of the salts in aqueous or other protic solvent based electrolytes, but not together with any of the standard aprotic solvents used for non-aqueous LIBs and SIBs.| Salts | Solvents | ||||
|---|---|---|---|---|---|
| Aprotic | Protic | ||||
| ACN | PC | EC:DMC | PEO | H2O | |
| Li | <0.01 | <0.01 | <0.01 | <1 | <6 |
| Na | <0.01 | <0.01 | <0.01 | <1 | <6 |
3.4. Thermal properties
A proper evaluation of the thermal stability is pivotal as a first safety test for any salt suggested for battery electrolyte usage, in order to assess its possible decomposition e.g. with respect to thermal runaway. The NaMM4411 salt decomposes at 315 °C, which is 10 °C lower than the LiMM4411 salt (Fig. 4). Thus, both our high purity fluorine-free salts exhibit higher Td:s than the most popular fluorine based salts such as sodium bis(fluorosulfonyl)imide (NaFSI),58,59 the lithium analogue (LiFSI),60 and lithium hexafluorophosphate (LiPF6),59 used in non-aqueous electrolytes,58–60 supporting our salts' excellent thermal stabilities.An analysis of DSC traces in principle allows identification of the presence of crystalline and amorphous phases via phase transitions. However, there are no thermal events related to neither the amorphous, glass transitions (Tg) nor the crystalline, melting (or solidification) (Tm), phases before the final decomposition for either of the salts (Fig. 5). This indicates quite large lattice energies of the present salts – again consistent with the crystallography data and the computational studies. The only features detectable are a small endothermic peak, at ca. 174 °C during heating, and a corresponding exothermic peak, at ca. 161 °C during cooling, for the NaMM4411 salt, with the areas of the peaks very close to identical; 6.1 (2.07) vs. 6.3 (2.14) J g−1 (kJ mol−1), respectively. A complementary experiment, heating on a hot plate, gives by visual inspection that this likely is related to a solid–solid phase transition.
3.5. Ionic interactions
As both the crystallography data and the computational study32 point to strong ion–ion interactions for the salts, we apply Raman and IR spectroscopy to in more detail probe these interactions. The symmetric and asymmetric stretching modes of the SO3− groups, νs(SO3−) and νas(SO3−), being sensitive to different environments,61,62 will be affected differently by the presence of the different alkali ions, Li+ and Na+ in the LiMM4411 and NaMM4411 salts, respectively.63,64 In the literature somewhat comparable sodium 1-alkanesulfonate salts and the disodium α,ω-alkanedisulfonates show strong bands related to νs(SO3−) between 1025 and 1080 cm−1 and the νas(SO3−) between 1167 and 1220 cm−1.65,66The νs(SO3−) is analyzed with Raman spectroscopy while the νas(SO3−) is investigated with IR spectroscopy as the latter only has weak bands in Raman.65 The Raman spectrum of NaMM4411 (Fig. 6a) shows two strong bands located at slightly lower wavenumbers, 1042 and 1050 cm−1, than for LiMM4411, 1048 and 1053 cm−1, quite similar to the differences in shifts observed for example for the νs(SO3−) in sulfosuccinate salts.67 The shift change of ca. 5 cm−1 when moving from Li+ to Na+ based systems is likely due to the stronger interaction of Li+ with the anion, consistent with computational predictions for selected anions for battery application.68 Since no water is detected in the spectra (not shown), we exclude the downshifts to be due to any HBs formed between the anion and water molecules, as previously found for hydrates of disodium α,ω-alkanedisulfonates.65 The reason for the two features for each salt in this region is likely ascribable to an in-phase and out-of-phase coupling of the symmetric stretching mode, as previously suggested for the bis(2-ethylhexyl)sulfosuccinate anion.67
The extent of the split both for the Na and Li based salts is large enough to allow the presence of two distinct bands. On the basis of the band assignment previously made in the literature,67 the in-phase vibration is located at higher wavenumbers than the out-of-phase vibration. From the Raman spectra performed on the dried polycrystalline salts, we cannot clearly state the exact coordination, mono- or bi-dentate, between the cation and the SO3− units, as no experimental data for the non-coordinated MM anion exists. However, it has been shown computationally,69 that mono- and bi-dentate Li+ coordination will produce an up-shift and a downshift, respectively, of the νs(SO3−) as compared to the “free” anion. Here, however, based also on the single crystal data, we can only assume that both the Li+ and Na+ coordination is monodentate to the SO3− groups. Moving to the IR spectra, the NaMM4411 salt again has peaks related to the νas(SO3−) located at lower wavenumbers (1156 and 1198 cm−1) than for the Li+ analogue (1171 and 1208 cm−1) (Fig. 6b).
In all, the sizes of the shifts are consistent with the difficulties encountered in solubilizing the salts in the aprotic solvents as well as consistent with the cation environments found in the crystallographic data.
3.6. Ionic conductivity
Analysis of the total ionic conductivity provides indication on the mobility of the ionic species in the electrolyte. The conductivity of the Na based aqueous electrolyte is higher than the corresponding Li based (Fig. S7†). This difference can be interpreted mainly in terms of the lower mobility of the Li+ hydrated ion when compared to Na+.70,71 However, also the lower interaction energies between the SO3− groups and the Na+ as compared with Li+ can play a role in the ionic mobility. The ionic conductivity of the NaMM4411 and LiMM4411 based electrolytes at 30 °C are ∼20 and 30 mS cm−1, respectively, close to the conductivity of an aqueous solution of LiTFSI at similar concentration,31 while LiTFSI is more soluble. However, at such concentrations, the conductivities are lower than those measured for aqueous electrolytes based on inorganic salts e.g. Na2SO4, Li2SO4.72,733.7. Electrochemical stability window (ESW)
The ESW determines the operating window and is also beneficial in providing a choice of feasible electroactive materials. The ESWs of the Li and Na based electrolytes (Fig. S8†) are about 2 V, −0.64 to 1.40 V vs. Ag/AgCl, and are limited by water decomposition: hydrogen evolution on the cathodic scan and oxygen evolution on the anodic scan. The ESWs are higher than what could be expected on the basis of the thermodynamic stability of water74 in agreement with similar aqueous electrolytes based on LiTFSI,75 Li2SO4 and LiNO3.764. Conclusions
Here we have reported on the synthesis and characterization of new Li and Na salts based on the first generation of “Mickey Mouse™” pseudo-delocalized anions. Both fluorine-free compounds are obtained in high purity and yield, by using readily available starting materials and water as reaction medium. Purification was carried out without the use of any columns, making the method attractive for future large scale production. X-ray crystallography on the two salts revealed their characteristics in the molecular arrangement, water incorporation, and (Lewis acid–base) interactions, all dependent on the metal ions. Strong interactions of the SO3− groups to the Li+ or Na+ ions, found in the crystals, explains in part the observed low solubility in aprotic solvents and high compatibility with water, consequently opening up for application of these salts especially in aqueous electrolyte. The ionic association study shows a weaker interaction between the MM anion and the Na+ ions than for the Li+ ions. We envision these salts to have a good combination of safety; thermally stable and lack of both F and P atoms in the structure, and environmentally-friendliness, not only during the synthesis, but also for future application in aqueous low voltage LIB and SIB electrolytes.Acknowledgements
The financial support by the Swedish Energy Agency through the “Batterifonden” research programme is gratefully acknowledged. The authors are also grateful of fruitful discussions with Prof. Michel Armand and to several of Chalmers Areas of Advance; Energy, Materials Science, and Transport for continuous support.References
- European Parliament Council, Official Journal of the European Union, 2006 Search PubMed.
- A. R. Kamali, Green Chem., 2016, 18, 1952–1964 RSC.
- H. Zou, E. Gratz, D. Apelian and Y. Wang, Green Chem., 2013, 15, 1183–1191 RSC.
- C. Zhu and T. Akiyama, Green Chem., 2016, 18, 2106–2114 RSC.
- X. Zhou, F. Chen, T. Bai, B. Long, Q. Liao, Y. Ren and J. Yang, Green Chem., 2016, 18, 2078–2088 RSC.
- K. Saravanan, M. Nagarathinam, P. Balaya and J. J. Vittal, J. Mater. Chem., 2010, 20, 8329–8335 RSC.
- K. Xu, Chem. Rev., 2014, 114, 11503–11618 CrossRef CAS PubMed.
- S. Wilken, M. Treskow, J. Scheers, P. Johansson and P. Jacobsson, RSC Adv., 2013, 3, 16359–16364 RSC.
- S. Wilken, P. Johansson and P. Jacobsson, Solid State Ionics, 2012, 225, 608–610 CrossRef CAS.
- S. E. Sloop, J. K. Pugh, S. Wang, J. B. Kerr and K. Kinoshita, Electrochem. Solid-State Lett., 2001, 4, A42–A44 CrossRef CAS.
- S. E. Sloop, J. B. Kerr and K. Kinoshita, J. Power Sources, 2003, 119–121, 330–337 CrossRef CAS.
- C. L. Campion, W. Li and B. L. Lucht, J. Electrochem. Soc., 2005, 152, A2327–A2334 CrossRef CAS.
- B. Ravdel, K. M. Abraham, R. Gitzendanner, J. DiCarlo, B. Lucht and C. Campion, J. Power Sources, 2003, 119–121, 805–810 CrossRef CAS.
- A. Hammami, N. Raymond and M. Armand, Nature, 2003, 424, 635–636 CrossRef CAS PubMed.
- L. Vimmerstedt, S. Ring and C. Hammel, Current status of environmental, health, and safety issues of lithium ion electric vehicle batteries, US Dept. of Energy, National Renewable Energy Lab, Golden, Colorado, 1995 Search PubMed.
- G. L. Henriksen, K. Amine, J. Liu and P. A. Nelson, Materials Cost Evaluation Report for High-Power Li-Ion HEV Batteries, US Dept. of Energy, 2002 Search PubMed.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- S. Wilken, PhD thesis, Chalmers University of Technology, 2014.
- J. Foropoulos Jr and D. D. Desmarteau, Inorg. Chem., 1984, 23, 3720–3723 CrossRef.
- L. Niedzicki, M. Kasprzyk, K. Kuziak, G. Z. Zukowska, M. Armand, M. Bukowska, M. Marcinek, P. Szczeciński and W. Wieczorek, J. Power Sources, 2009, 192, 612–617 CrossRef CAS.
- J. Scheers, D.-H. Lim, J.-K. Kim, E. Paillard, W. A. Henderson, P. Johansson, J.-H. Ahn and P. Jacobsson, J. Power Sources, 2014, 251, 451–458 CrossRef CAS.
- M. Yoshizawa and H. Ohno, Ionics, 2002, 8, 267–271 CrossRef CAS.
- M. Egashira, B. Scrosati, M. Armand, S. Béranger and C. Michot, Electrochem. Solid-State Lett., 2003, 6, A71–A73 CrossRef CAS.
- P. Johansson, S. Béranger, M. Armand, H. Nilsson and P. Jacobsson, Solid State Ionics, 2003, 156, 129–139 CrossRef CAS.
- A. Bitner, G. M. Nolis, T. Trzeciak, G. A. Źukowska, L. Niedzicki, W. Wieczorek and M. Marcinek, ECS Meeting Abstracts, 2015, vol. MA2015–01, p. 411 Search PubMed.
- H. Kim, J. Hong, K. Y. Park, H. Kim, S. W. Kim and K. Kang, Chem. Rev., 2014, 114, 11788–11827 CrossRef CAS PubMed.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- Y. Wang, J. Yi and Y. Xia, Adv. Energy Mater., 2012, 2, 830–840 CrossRef CAS.
- W. Li, J. R. Dahn and D. S. Wainwright, Science, 1994, 264, 1115–1118 CrossRef CAS PubMed.
- L. Suo, O. Borodin, T. Gao, M. Olguin, J. Ho, X. Fan, C. Luo, C. Wang and K. Xu, Science, 2015, 350, 938–943 CrossRef CAS PubMed.
- E. Jónsson, M. Armand and P. Johansson, Phys. Chem. Chem. Phys., 2012, 14, 6021–6025 RSC.
- E. Jónsson, M. B. Armand and P. Johansson, US Pat. 9,269,987, 2016.
- Z. Liu, X. Ouyang, R. Peng, Y. Bai, D. Mi, W. Jiang, A. Facchetti and Z. Ge, J. Mater. Chem. A, 2016, 4, 2530–2536 RSC.
- C. Wang, X. Liu, M. Yang, H. Ma, P. Yan, J. M. Slattery and Y. Gao, RSC Adv., 2013, 3, 8796–8804 RSC.
- Bruker, SADABS, Bruker AXS Inc., Madison, Wisconsin, USA, 2007 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- C. B. Hübschle, G. M. Sheldrik and B. Dittrich, J. Appl. Crystallogr., 2011, 44, 1281–1284 CrossRef PubMed.
- F. J. Harris, Proc. IEEE, 1978, 66, 51–83 CrossRef.
- J. F. King and S. Skonieczny, Phosphorus Sulfur Relat. Elem., 1985, 25, 11–20 CrossRef CAS.
- L. Sonnenschein and A. Seubert, Tetrahedron Lett., 2011, 52, 1101–1104 CrossRef CAS.
- S. Osterman-Golkar and C. A. Wachtmeister, Chem.-Biol. Interact., 1976, 14, 195–202 CrossRef CAS PubMed.
- L. Xue, C. W. Padgett, D. D. DesMarteau and W. T. Pennington, Solid State Sci., 2002, 4, 1535–1545 CrossRef CAS.
- J. L. Allen, S.-D. Han, P. D. Boyle and W. A. Henderson, J. Power Sources, 2011, 196, 9737–9742 CrossRef CAS.
- T. V. Hoogerstraete, N. R. Brooks, D. Onghena, L. V. Meervelt and K. Binnemans, CrystEngComm, 2015, 17, 7142–7149 RSC.
- A. P. Marczewska, T. Trzeciak, A. Bitner, L. Niedzicki, M. Dranka, G. Z. Zukowska, M. Marcinek and W. Wieczorek, Chem. Mater., 2014, 26, 4908–4914 CrossRef.
- J. D. Holbrey, W. M. Reichert, M. Nieuwenhuyzen, S. Johnson, K. R. Seddon and R. D. Rogers, Chem. Commun., 2003, 1636–1637 RSC.
- T. Mandai, H. Masu, H. Seki and K. Nishikawa, Bull. Chem. Soc. Jpn., 2012, 85, 599–605 CrossRef CAS.
- M. H. Lamers, A. Perrakis, J. H. Enzlin, H. H. K. Winterwerp, N. Wind and T. K. Sixma, Nature, 2000, 407, 711–717 CrossRef CAS PubMed.
- R. Centore and A. Tuzi, Cryst. Eng., 2003, 6, 87–97 CrossRef CAS.
- K. Goossens, K. Lava, C. W. Bielawski and K. Binnemans, Chem. Rev., 2016, 116, 4643–4807 CrossRef CAS PubMed.
- W. Ji, G. Liu, Z. Li and C. Feng, ACS Appl. Mater. Interfaces, 2016, 8, 5188–5195 Search PubMed.
- T. Mandai, H. Masu and P. Johansson, Dalton Trans., 2015, 44, 11259–11263 RSC.
- J. L. M. Abboud and R. Notario, Pure Appl. Chem., 1999, 71, 645–718 CrossRef CAS.
- B. L. Papke, M. A. Ratner and D. F. Shriver, J. Phys. Chem. Solids, 1981, 42, 493–500 CrossRef CAS.
- L. Edman, A. Ferry and M. M. Doeff, J. Mater. Res., 2000, 15, 1950–1954 CrossRef CAS.
- A. Boschin and P. Johansson, Electrochim. Acta, 2015, 175, 124–133 CrossRef CAS.
- K. Kubota, T. Nohira and R. Hagiwara, J. Chem. Eng. Data, 2010, 55, 3142–3146 CrossRef CAS.
- G. G. Eshetu, S. Grugeon, H. Kim, S. Jeong, L. M. Wu, G. Gachot, S. Laruelle, M. Armand and S. Passerini, ChemSusChem, 2016, 9, 462–471 CrossRef CAS PubMed.
- M. Kerner, N. Plylahan, J. Scheers and P. Johansson, RSC Adv., 2016, 6, 23327–23334 RSC.
- N. B. Colthup, L. H. Daly and S. E. Wiberley, Introduction to Infrared and Raman Spectroscopy, Elsevier Science, 1990 Search PubMed.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, Wiley, 2004 Search PubMed.
- A. Brodin, B. Mattsson, K. Nilsson, L. M. Torell and J. Hamara, Solid State Ionics, 1996, 85, 111–120 CrossRef CAS.
- R. J. Capwell, K. H. Rhee and K. S. Seshadri, Spectrochim. Acta, Part A, 1968, 24, 955–958 CrossRef CAS.
- K. Ohno, T. Naganobu, H. Matsuura and H. Tanaka, J. Phys. Chem., 1995, 99, 8477–8484 CrossRef CAS.
- A. Nersasia and P. R. Johnson, J. Appl. Polym. Sci., 1965, 9, 1653–1668 CrossRef.
- P. D. Moran, G. A. Bowmaker, R. P. Cooney, J. R. Bartlett and J. L. Woolfrey, J. Mater. Chem., 1995, 5, 295–302 RSC.
- E. Jónsson and P. Johansson, Phys. Chem. Chem. Phys., 2012, 14, 10774–10779 RSC.
- S. P. Gejji, K. Hermansson, J. Tegenfeldt and J. Lindgren, J. Phys. Chem., 1993, 97, 11402–11407 CrossRef CAS.
- K. Fic, G. Lota, M. Meller and E. Frackowiak, Energy Environ. Sci., 2012, 5, 5842–5850 Search PubMed.
- C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang and J. Zhang, Chem. Soc. Rev., 2015, 44, 7484–7539 RSC.
- A. Cartón, F. Sobrón, S. Bolado and J. I. Gerbolés, J. Chem. Eng. Data, 1995, 40, 987–991 CrossRef.
- R. V. Lundquist and R. W. Lewis, J. Chem. Eng. Data, 1957, 2(1), 69–72 CrossRef CAS.
- M. Pourbaix, Atlas of Electrochemical Equilibria in Aqueous Solutions, NACE, Houston TX, 2nd edn, 1974 Search PubMed.
- S. F. Lux, L. Terborg, O. Hachmöller, T. Placke, H.-W. Meyer, S. Passerini, M. Winter and S. Nowak, J. Electrochem. Soc., 2013, 160(10), A1694–A1700 CrossRef CAS.
- C. Wessells, R. Ruffo, R. A. Huggins and Y. Cui, Electrochem. Solid-State Lett., 2010, 13, A59–A61 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1492164 and 1492165. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra19623c |
| ‡ These authors have contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2016 |