A novel graphene oxide coated biochar composite: synthesis, characterization and application for Cr(vi) removal
Abstract
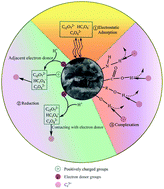
In the current work, a graphene oxide coated water hyacinth biochar composite (WHB-GO) was synthesized to remove Cr(VI) from aqueous solution. The biomass feedstock was firstly treated with graphene oxide and then annealed at 300 °C in a quartz tube furnace under N2 atmosphere. After synthesis, full characterization with various techniques (SEM, FT-IR, XPS and BET) were used to analyze the properties of the adsorbent and the sorption mechanisms of Cr(VI). The effects of pH, ionic strength, sorption kinetics, isotherms and thermodynamics, as well as comparison and regeneration experiments were also investigated. The results indicated that the adsorption capacity was significantly influenced by pH and ionic strength. The maximum adsorption capacity (150.02 mg g−1) of the WHB-GO was obtained at pH 2.0 and 50 °C. Besides, the sorption data could be fitted well by pseudo-second-order and Freundlich models. The thermodynamic studies indicated that the adsorption reaction was a spontaneous and endothermic process. The enhanced adsorption of Cr(VI) on the WHB-GO was mainly controlled by electrostatic attraction and reduction of Cr(VI) coupled with Cr(III) complexation. The regeneration study revealed that WHB-GO could be reused almost six times without loss of activity in adsorption tests. Overall, WHB-GO can be used as a novel, facile, and low-cost sorbent for the removal of Cr(VI) from aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...