MCM-41-immobilized 1,10-phenanthroline–copper(i) complex: a highly efficient and recyclable catalyst for the coupling of aryl iodides with aliphatic alcohols†
Abstract
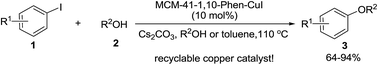
A heterogeneous C–O coupling reaction between aryl iodides and aliphatic alcohols was achieved in neat alcohol or toluene at 110 °C in the presence of 10 mol% of the MCM-41-immobilized 1,10-phenanthroline–copper(I) complex [MCM-41-1,10-phen–CuI] with Cs2CO3 as a base, yielding a variety of aryl alkyl ethers in good to excellent yields. The new heterogeneous copper catalyst can easily be prepared by a simple procedure from commercially available and inexpensive reagents, and recovered by filtration of the reaction solution and recycled at least 8 times without significant loss of activity.

 Please wait while we load your content...
Please wait while we load your content...