A novel facile and fast hydrothermal-assisted method to synthesize sulfur/carbon composites for high-performance lithium–sulfur batteries†
Abstract
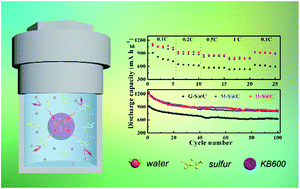
Hydrothermal-assisted sulfur impregnation method was first proposed to prepare sulfur/carbon (S/C) composites for lithium–sulfur (Li–S) battery applications. Comparing with the currently existing sulfur impregnation method, this facile one-pot method is proved to be energy-saving and time-saving, to have a sulfur content that is exactly controllable and to be environment-friendly. In the hydrothermal environment, sulfur would selectively diffuse into the pores of carbon hosts due to its high mobility, homogeneous dispersibility, hydrophobicity and carbon affinity. As a result, the S/C composite obtained from hydrothermal-assisted method under a low temperature of 120 °C and a short time of 2 hours exhibits a comparable battery performance to that obtained from the traditional melting method under 155 °C for 20 hours, which reached 1239 mA h g−1 at 0.2C and 796 mA h g−1 at even 1C between 1.85–2.8 V, being rather suitable for large-scale manufacture and commercial development.

 Please wait while we load your content...
Please wait while we load your content...