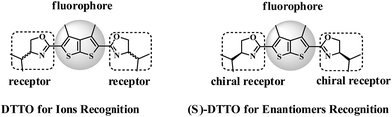
Design, synthesis and fluorescence behavior of novel chemosensor with a thieno[2,3-b]thiophene fluorophore
Jing Cao*,
Wanghui Yan and
Yiling Huang
Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan 411105, People's Republic of China. E-mail: caojing8088@xtu.edu.cn
First published on 17th October 2016
Abstract
A novel fluorescent chemosensor, a thieno[2,3-b]thiophene derivative carrying two oxazoline groups (DTTO) was designed and synthesized, which was discovered to exhibit good selectivity to dichromate anions (Cr2O72−). We also found that if the oxazoline group was replaced with a chiral one, for example, (S)-DTTO acted as a chiral fluorescent chemosensor, which exhibited a distinguishing fluorescent response to the enantiomers of mandelic acid.
Introduction
Sensor molecules responding to substrates present a sensitive optical or electric method for monitoring molecular interactions.1,2 Such sensors are playing increasing roles in analytical fields such as biology, medicine and the environment.3–5 Enantioselective optical sensors for chiral compounds6–8 and monitoring oxidizers with electrochemical sensors have attracted great interest in recent years.9–12The optical chemosensor such as fluorescent chemosensor is often applied in enantioselective recognition, generally containing a fluorophore and a receptor. The fluorophore generally requires high absorption and emission in ultraviolet or visible region. The receptor of chemosensor often has ability of interaction with the monitored species, by which the process of photoinduced electron/energy transfer (PET),13 metal–ligand charge transfer (MLCT)14 or intramolecular charge transfer (ICT) occur.15
Electrochemical sensor is often used in monitoring oxidizers since the change of electrochemical properties is easily monitored during the reduction process of the oxidizers. However, sensing the process via optical method is not very common.
Herein, we report a kind of novel fluorescent chemosensor DTTO, in which oxazoline motifs bear nitrogen and oxygen coordination atom acting as the receptor. Here the rich electron polycyclic aromatic 3,4-dimethylthieno[2,3-b]thiophene is chosen as the fluorophore, since it has strong absorption or emission band in ultraviolet region.16,17 The recognition behavior of DTTO (6) for ions was checked. We found it could be acted as fluorescent chemosensor for the oxidizer ion Cr2O72−. Moreover, 3,4-dimethylthieno[2,3-b]thiophene fluorophore motif with chiral oxazoline receptors (7) ((S)-DTTO) was found to be an optical sensor for enantiomers (Scheme 1).
Results and discussion
Synthesis of DTTO and (S)-DTTO
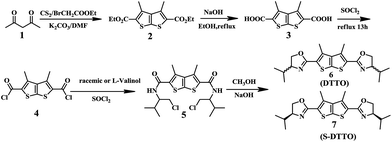
DTTO or (S)-DTTO was synthesized according the route in Scheme 2. Acetylacetone, carbondisulfide and ethyl bromoacetate were used as the starting material, over two steps, resulted in the desired 3,4-dimethylthieno[2,3-b]thiophene-2,5-dicarboxylic acid 3.18 The dicarbonyl chloride 4 was produced by treatment of 3 with SOCl2, and then addition of valinol and triethylamine to the solution of 4 gave 2,5-bis(1-chloride-3-methylbutan-2-yl)-3,4-dimethylthieno[2,3-thiophene 5 with 39% yield. Finally, NaOH was used in methanol to close ring, affording the target product 6 or 7 in 64% yield.Optical behavior of DTTO as a chemosensor
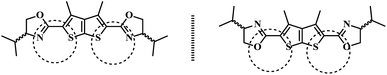
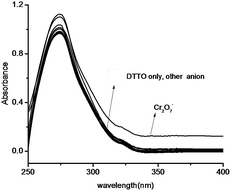
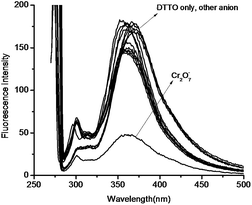
DTTO is firstly designed as a PET fluorescent chemosensor for metal ions based on the following considerations. Thieno[2,3-b]thiophene motif, 10 π electronic aromatic compound, has achieved much attention as π-electron donor due to its good optical and electrical properties.19 It is rarely exploited as a fluorophore of chemosensor that stimulate our great interest. Selecting oxazoline as receptor on account of this unit containing nitrogen and oxygen coordination atom on five heterocycle,20 which may possess ability of complexation with metal ions if it can cooperate with sulfur atoms on thieno[2,3-b]thiophene motif (Scheme 3). Moreover, oxazolines with chiral substituent are conveniently accessible from commercially available chiral β-amino alcohols.21 Thus, the corresponding chemosensor might be applied in enantiomers recognition.Therefore, the recognition of DTTO to metal ions has been investigated in priority. We tested the change of fluorescence intensity of DTTO in the presence of metal ions. The alkali metal cations (Li+, Na+, K+), alkaline-earth metal cations (Mg2+, Ca2+, Sr2+, Ba2+) and heavy and transition metal (HTM) cations (Ni2+, Mn2+, Co2+, Cd2+, Hg2+) were used to evaluate the binding behavior. However, when different metal ions (100 eq.) were added in the acetonitrile solution of the sensor 6, no obvious fluorescent change of sensor have happened in these alkaline metal ions, alkaline-earth metal ions and heavy and transition metal cations. The experimental data indicate that 6 is not a highly sensitive and selective sensor for metal ions in acetonitrile solution. These results suggest that complex ability and binding property of 6 for metal ions are relatively weak. It could be explained that sulfur atoms on thiophene motif has poor coordinate interaction due lone pair electrons of sulfur participating in conjugation. The UV-vis absorption detection selectivity of DTTO adding the common anions (50 eq.) such as BrO3−, F−, I−, IO3−, NO3−, SCN−, CO32−, Cr2O72−, SO32−, SO42−, PO43−, Cl−, H2PO4−, HCO3−, OH− was then investigated (Fig. 1). The absorption spectra of DTTO shows a main absorption band with λmax of 275 nm. The absorption spectra does not show much difference when the anions added respectively. Only Cr2O72− display weak enhancement after 300 nm. It indicates that DTTO may exhibit selectivity toward Cr2O72−.
Next, fluorescence emission behaviors of DTTO in the presence of various anions were recorded. Fig. 2 shows the fluorescence spectra (λex = 275 nm) of DTTO measured in acetonitrile with each respective anion (50 eq.). We found that the fluorescence of DTTO nearly has no significant effect in the presence of BrO3−, F−, I−, IO3−, NO3−, SCN−, CO32−, Cr2O72−, SO32−, SO42−, PO43−, Cl−, H2PO4−, HCO3−, OH− ions. However, under the same conditions, Cr2O72− exhibit greatly fluorescence quenching. The fluorescence intensity. Thus, DTTO could act as a Cr2O72− selective supramolecular fluorescence probe.
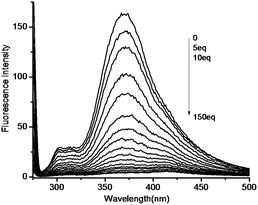
Then we investigated the sensitivity of DTTO towards Cr2O72− ion in acetonitrile solutions in details (Fig. 3). The gradual decrease in its fluorescent intensities could be obviously observed with the ratio changes of Cr2O72− ion from 0 to 150 eq., especially at the high concentration of Cr2O72− ion. In Fig. 5, it is easy to find when fluorescence of DTTO can be completely quenched when the ratio of Cr2O72− ion reach 100 eq.
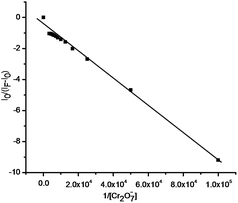
On the basis of the fluorescence titrations of DTTO, the association constant Ka was evaluated graphically by the Benesi–Hildebrand plot (Fig. 4). The data was linearly fit according to the Benesie–Hildebrand equation and the Ka value was obtained from the slope and intercept of the line. (Fig. 4: Y = A + BX, Ka = A/B, X = 1/[Cr2O72−], Y = I0/(IF − I0); Ka: the association constant, I0: the fluorescent intensity of DTTO, IF: the fluorescent intensity of DTTO-complex; A = −0.5837, B = −8.53295 × 10−5, R2 = 0.99285, Ka = A/B = 6.8 × 103). In order to understand the interaction stoichiometry of DTTO–Cr2O72−, job plot experiments were carried out.
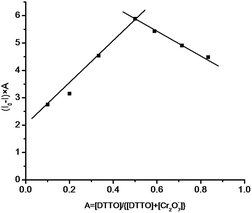
In Fig. 5, the emission intensity at 375 nm is plotted against molar fraction of DTTO under a constant total concentration. In the case of the complexation with Cr2O72−, the complex approached a maximum when the mole fraction of guest ca. 0.5, suggesting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest interaction. We proposed that there might be an interaction between two sulfur atoms of thieno[2,3-b]thiophene moiety and Cr2O72−, in this case, 1
1 host–guest interaction. We proposed that there might be an interaction between two sulfur atoms of thieno[2,3-b]thiophene moiety and Cr2O72−, in this case, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexation may occur. The mechanism of fluorescence quenching between DTTO and Cr2O72− might be resulted from the oxidation of Cr2O72− to sulfur atoms because thieno[2,3-b]thiophene is a electron-rich polycyclic aromatic ring which is extremely attacked by electron-defect species. Based on these results, thieno[2,3-b]thiophene derivatives are promised to become new fluorescent chemosensor for monitoring harmful oxidant in environment.
1 host–guest complexation may occur. The mechanism of fluorescence quenching between DTTO and Cr2O72− might be resulted from the oxidation of Cr2O72− to sulfur atoms because thieno[2,3-b]thiophene is a electron-rich polycyclic aromatic ring which is extremely attacked by electron-defect species. Based on these results, thieno[2,3-b]thiophene derivatives are promised to become new fluorescent chemosensor for monitoring harmful oxidant in environment.
Enantiomers recognition of (S)-DTTO
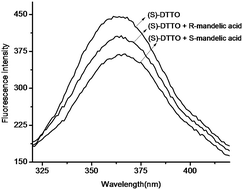
If the oxazoline motifs of DTTO are displaced by chiral ones, for example, (S)-DTTO could be as a fluorescent chemosensor for enantiomers recognition. The interactions of (S)-DTTO with several enantiomers, such as mandelic acid, 1,1′-binaphthol (BINOL) and phenylalanine have been studied. The emission spectrum of (S)-DTTO in acetonitrile were recorded (excitation at 275 nm). Adding the enantiomers into the acetonitrile solution of (S)-DTTO respectively, almost no difference between the effect of (R)- and (S)-BINOL, (R)- and (S)-phenylalanine were found. However, an obvious difference was observed for the fluorescence response at 365 nm toward (R)- and (S)-mandelic acid. As showed by Fig. 6, (R)-mandelic acid (50 eq.) decrease the fluorescence of (S)-DTTO (2 × 10−6 M in acetonitrile solution), and (S)-mandelic acid also decreased the fluorescence intensity of (S)-DTTO. But there is a obvious distinction between them. The decreased fluorescence intensity of (S)-DTTO with (S)-mandelic acid (from 445 a.u. to 367 a.u.), is nearly one times than (R)-mandelic acid (from 445 a.u. to 405 a.u.).In order to ascertain that the observed large difference in the fluorescence responses of (S)-DTTO toward (R)- and (S)-mandelic acid is due to an inherent chiral recognition. A further investigation on the fluorescence response of (S)-DTTO with different amount of mandelic acid enantiomers was performed. Fluorometric titrations were carried out by addition of known quantities of mandelic acid to 2 × 10−6 M solution of free ligand (Fig. 7). Titration of (R) or (S)-mandelic acid into acetonitrile solution of (S)-DTTO both give a concomitant decrease of the fluorescence intensity, which hints that there is the stronger interaction between (S)-mandelic acid and (S)-DTTO. It also demonstrates that the fluorescence interaction of (S)-DTTO with mandelic acid is indeed enantioselective.
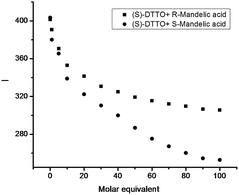 | ||
| Fig. 7 The difference fluorescence responses of (S)-DTTO (2 × 10−6 M) between enantiomers of mandelic acid with different concentration. | ||
As is well known that chiral recognition requires multiple-point interaction,22 we thus envisioned that through an additional interaction, e.g., hydrogen binding, mono α-hydroxyl acids could be enantioselective toward (S)-DTTO. However, it is difficult to find direct evidence of the interaction model between (S)-DTTO and mandelic acid. But we calculated total energy of (S)-DTTO + (S)-mandelic acid and (S)-DTTO + (R)-mandelic acid respectively when put DTTO and mandelic acid molecules together, using Chem3D Ultra 8.0 programme to minimize system energy with MM2 method. The total energy of (S)-DTTO + (R)-mandelic acid (46.5 kcal mol−1) is obviously larger than that of (S)-DTTO + (S)-mandelic acid (44.4 kcal mol−1). Though the interaction model is not yet clear, we could draw the conclusion that (S)-DTTO is easier to interact with (S)-mandelic acid than (R)-mandelic acid.
Conclusions
In summary, a novel 3,4-dimethylthieno[2,3-b]thiophene derivative bearing two oxazoline groups at 2- and 5-positions (DTTO) exhibits selectivity to anion Cr2O72− via fluoresce emission change due to oxidation of electron-rich thieno[2,3-b]thiophene motif, which may be a new type of fluorescent chemosensor for monitoring the trace danger oxidizer in environment. In addition, the optical active (S)-DTTO displays a fluorescence enantioselective toward to mandelic acid.Materials and instruments
Commercial chemical reagents were obtained without further purification from suppliers of Aladdin Reagent (Shanghai, China) or Acros Company. The analytical purity inorganic salts were used. Both of absorption and fluorescence titrations were performed with a concomitant addition of small volumes of aqueous ion solution or enantiomers acetonitrile solution to acetonitrile solution of DTTO or (S)-DTTO. 1H NMR and 13C NMR were checked on a Bruker AVANCE 400 MHz spectrometer with chemical shifts reported in ppm (CDCl3, TMS as internal standard). Mass spectrum was tested on Agilent 6120 instrument. UV-visible spectra were taken on a Lambda 25 spectrophotometer. Fluorescence spectra were scanned on a Perkin-Elmer LS55 luminescence spectrometer with a xenon lamp as the light source, 10/10 nm as excitation and emission slit and 275 nm as excitation.General procedure for synthesis of compound DTTO and S-DTTO
To a solution of racemic valinol (3.72 g, 36 mmol) and triethylamine (14 ml) in CHCl3 (65 ml) was added a solution of the acid chloride 4 in CHCl3 (65 ml) at 0 °C. The mixture was stirred for 1 day at r.t. and then SOC12 (15 ml) was added. After stirred for 3 h, the mixture was poured into ice-water (250 ml) and extracted with CH2Cl2. The extract was washed with brine, dried over Na2SO4, removing solvent gave white solid product which was purified by silica gel column chromatography with CH2Cl2 as eluent. Yield: 39%. 1H NMR (400 MHz, CDCl3) δ 5.94 (s, 1H; –NH), 4.39 (s, 1H; –CH), 4.13 (s, 2H; –CH2), 3.80 (s, 1H; –CH), 2.79 (s, 3H; –CH3), 0.94 (d, J = 6.7 Hz, 6H; –CH3).To a solution of the bis-amidochloride 5 (3.6 g, 8.0 mmol) in methanol (80 ml) was added aqueous NaOH (2.5 M, 20 ml). The mixture was stirred for 37 h at 40 °C. The mixture was extracted with CH2Cl2, dried over Na2SO4 and concentrated. The residue was purified by silica gel column chromatography with CH2Cl2 as eluent giving DTTO (6). Yield: 64%. [α]436 = −53.5 (20 °C, c = 2 g L−1 in CH2Cl2) 1H NMR (400 MHz, CDCl3) δ 4.37 (s, 1H; –CH), 4.10 (s, 2H; –CH2), 2.83 (s, 3H; –CH3), 1.84 (d, J = 5.7 Hz, 1H; –CH), 0.98 (dd, J = 36.8, 5.8 Hz, 6H; –CH3). 13C NMR (100 MHz, DMSO-d6) δ 159.38 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 136.12, 127.01, 72.70, 70.04 (thiofuran), 32.96 (N–CH), 29.73 (O–CH2), 18.90 (CH), 18.22 (–CH3), 14.54 (–CH3). MS (ESI) m/z: 390.9 (M + 1).
N), 136.12, 127.01, 72.70, 70.04 (thiofuran), 32.96 (N–CH), 29.73 (O–CH2), 18.90 (CH), 18.22 (–CH3), 14.54 (–CH3). MS (ESI) m/z: 390.9 (M + 1).
L-Valinol was used to replace the racemic one in the preparative procedure, the final product 2,5-bis((S)-4-isopropyloxazolin-2-y1)-3,4-dimethylthieno[2,3-b]thiophene (S-DTTO (7)) was obtained with the same yield as compound 6 except the value of specific rotation is −53.5 (20 °C, 436 nm, c = 2 g L−1 in CH2Cl2).
Acknowledgements
We thank Hunan Provincial Natural Science Foundation of China (no. 2016JJ6147) for financial support.Notes and references
- A. P. deSilva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, G. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef CAS.
- F. Sancenón, R. Martínez-Máñez, M. A. Miranda and M. J. Seguí, Angew. Chem., 2003, 42, 647 CrossRef PubMed.
- E. J. Song, H. Kim, H. Hwang, K. B. Kim and A. R. Kim, Sens. Actuators, B, 2014, 195, 36 CrossRef CAS.
- Y. Yang, X. L. Ni, T. Sun, H. Cong and G. A. Wei, RSC Adv., 2014, 87, 47000 RSC.
- Y. Li, K. Li and J. He, Talanta, 2016, 153, 381 CrossRef CAS PubMed.
- M. G. Finn, Chirality, 2002, 14, 534 CrossRef CAS PubMed.
- Z. B. Li, J. Lin, Y. C. Qin and L. Pu, Org. Lett., 2005, 7, 3441 CrossRef CAS PubMed.
- G. A. Hembury, V. V. Borovkov and Y. Inoue, Chem. Rev., 2008, 108, 1 CrossRef CAS PubMed.
- X. Niu, W. Yang, G. Wang, J. Ren, H. Guo and J. Gao, Electrochim. Acta, 2013, 98, 167 CrossRef CAS.
- Y. Yin, J. Yi, S. Tang and S. Yang, Sens. Lett., 2015, 13, 592 CrossRef.
- Z. Wang, J. Yi and S. Yang, Sens. Actuators, B, 2013, 176, 211 CrossRef CAS.
- Y. Zhou, C. Chen, J. Zhao, J. Fei, Y. Ding and Y. Cai, Electrochim. Acta, 2016, 192, 158 CrossRef CAS.
- T. Gunnlaugsson, A. P. Davis, J. E. O'Brien and M. Glynn, Org. Lett., 2002, 4, 2499 CrossRef.
- P. D. Beer, Acc. Chem. Res., 1998, 31, 71 CrossRef CAS.
- V. Thiagarajan and P. Ramamurthy, J. Lumin., 2007, 126, 886 CrossRef CAS.
- Y. X. Wu, J. Cao, H. Y. Deng and J. X. Feng, Spectrochim. Acta, Part A, 2011, 82, 340 CrossRef CAS PubMed.
- J. Cao, H. Y. Deng, C. H. Wang, Y. Xiao, M. Ren and Y. W. Zhang, Supramol. Chem., 2011, 23, 777 CrossRef CAS.
- A. Comel and G. J. Kirsch, Heterocycl. Chem., 2001, 38, 1167 CrossRef CAS.
- M. C. Gather, M. Heeney, W. Zhang, K. S. Whitehead, D. D. C. Bradley, I. McCulloch and A. J. Campbell, Chem. Commun., 2008, 9, 1079 RSC.
- P. Wipf and C. P. Miller, J. Am. Chem. Soc., 1992, 114, 10975 CrossRef CAS.
- H. Brunner and U. Obermann, Chem. Ber., 1989, 122, 499 CrossRef CAS.
- L. Salem, X. Chapuisat, G. Segal, P. C. Hiberty, C. Minot, C. Leforestier and P. J. Sautet, J. Am. Chem. Soc., 1987, 109, 2887 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |