Oleiferasaponin C6 from the seeds of Camellia oleifera Abel.: a novel compound inhibits proliferation through inducing cell-cycle arrest and apoptosis on human cancer cell lines in vitro†
Jianfa Zonga,
Dongxu Wanga,
Weiting Jiaoa,
Liang Zhang a,
Guanhu Baoa,
Chi-Tang Hob,
Ruyan Hou*a and
Xiaochun Wan*a
a,
Guanhu Baoa,
Chi-Tang Hob,
Ruyan Hou*a and
Xiaochun Wan*a
aState Key Laboratory of Tea Plant Biology and Utilization, Anhui Agricultural University, Hefei 230036, China. E-mail: hry@ahau.edu.cn; xcwan@ahau.edu.cn; Fax: +86-551-65786765; Tel: +86-551-65786765
bDepartment of Food Science, Rutgers University, 65 Dudley Rd., New Brunswick, NJ 08901, USA
First published on 19th September 2016
Abstract
A new oleanane-type saponin oleiferasaponin C6 (OSC6) was isolated and identified from Camellia oleifera seeds through NMR, HR-ESI-MS, and GC-MS spectroscopic methods. This new oleanane-type saponin exhibits potent cytotoxic activities on five human cancer cell lines (BEL-7402, BGC-823, MCF-7, HL-60 and KB), especially in HL-60 and BEL-7402 cell lines. The action mechanism of the anti-cancer activity of OSC6 was further investigated and it showed significant induction of cell cycle arrest and apoptosis in HL-60 and BEL-7402 cell lines by down-regulating CDK4, cyclin D1 and Bcl-2; up-regulating p21, caspase-3/9, p-p53 and Bax protein expression in vitro. These results suggested that the newly isolated saponin OSC6 is a potential therapeutic agent for the treatment of human cancer.
Introduction
Cancer is a significant and growing cause of mortality worldwide, with an increase to 19.3 million new cancer cases per year projected for 2025.1,2 Stomach, liver and breast cancers are three common types of cancer in most countries. In addition, oral cancer and leukemia have emerged as significant, global public health concerns.3,4 In spite of the extensive efforts and investment in research, there remains an urgent need for development of more efficient anticancer agents with minimal side effects. Therefore, there is considerable interest in the discovery and development of novel phytochemicals, which has led to investigation of the crude plant extracts that exhibit broad-spectrum anti-tumor activity in certain tumors. Triterpenoid saponins, constitute an important group of plant secondary metabolites that show diverse pharmacological and biological activities.5–8 Because of the growing realization that triterpenoids may be a source of medication for cancer treatment, saponins have been extracted from Camellia oleifera Abel. plant.9–11C. oleifera Abel. is widely cultivated throughout southern China for the edible oil within its seeds. Approximately 1 million tons of the seeds yielding 730![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of defatted seeds are generated yearly in China. It should be noted that the defatted seeds contain about 10% saponins by dry weight,12 comprising more than 30 types of saponins.13,14 Although the seed saponins are difficult to purify and identify, recent studies of the structures and functions of saponins showed that there are 11 types of triterpenoid saponins, including 10 newly isolated types,10–12,15–18 and that some exhibited broad-spectrum, anti-proliferative effects at the micromole level against human lung, gastric, breast, liver and colon tumor cell lines.10–12 However, the inhibitory mechanisms of the saponins on cancer cells have not been widely reported yet. As a part of our ongoing study of the constituents of the C. oleifera seed, we recently isolated a new oleanane-type saponin from the C. oleifera seeds that showed better anti-cancer activities compared to other six saponins we previous identified (Fig. S1†).10,11 In the present study, we report the isolation and structural elucidation of this new saponin, namely oleiferasaponin C6 (OSC6), along with possible molecular mechanisms underlying its effects on multiple human cancer cell lines. We observed that OSC6 significantly inhibited proliferation of human hepatocellular carcinoma (BEL-7402) and human promyelocytic leukemia (HL-60) cells. Moreover, OSC6 induced cell apoptosis and led to a significant down-regulation of anti-apoptotic protein Bcl-2, up-regulation of pro-apoptotic protein Bax, and activation of the p53 pathway. Our results have provided the theoretical basis for the development of OSC6 as a potential novel anticancer agent.
000 tons of defatted seeds are generated yearly in China. It should be noted that the defatted seeds contain about 10% saponins by dry weight,12 comprising more than 30 types of saponins.13,14 Although the seed saponins are difficult to purify and identify, recent studies of the structures and functions of saponins showed that there are 11 types of triterpenoid saponins, including 10 newly isolated types,10–12,15–18 and that some exhibited broad-spectrum, anti-proliferative effects at the micromole level against human lung, gastric, breast, liver and colon tumor cell lines.10–12 However, the inhibitory mechanisms of the saponins on cancer cells have not been widely reported yet. As a part of our ongoing study of the constituents of the C. oleifera seed, we recently isolated a new oleanane-type saponin from the C. oleifera seeds that showed better anti-cancer activities compared to other six saponins we previous identified (Fig. S1†).10,11 In the present study, we report the isolation and structural elucidation of this new saponin, namely oleiferasaponin C6 (OSC6), along with possible molecular mechanisms underlying its effects on multiple human cancer cell lines. We observed that OSC6 significantly inhibited proliferation of human hepatocellular carcinoma (BEL-7402) and human promyelocytic leukemia (HL-60) cells. Moreover, OSC6 induced cell apoptosis and led to a significant down-regulation of anti-apoptotic protein Bcl-2, up-regulation of pro-apoptotic protein Bax, and activation of the p53 pathway. Our results have provided the theoretical basis for the development of OSC6 as a potential novel anticancer agent.
Materials and methods
General experimental procedures
UV (ultraviolet) spectra were recorded on a Hitachi U-5100 spectrophotometer. IR (infrared) spectra were measured on a Nicolet 8700 FT-IR spectrophotometer with KBr pellets. NMR spectra were obtained on an Agilent DD2 (600 MHz) spectrometer using pyridine-d5 as solvent. High resolution electro-spray ionization mass spectrometry (HR-ESI-MS) was run on an Electrostatic Field Orbital Trap Mass Spectrometer (Thermo Scientific). Semi-preparative HPLC was performed on a Varian Prostar HPLC Model 325 instrument (Varian, Mulgrave, Australia) and a YMC-Pack ODS-A semi-preparative HPLC column (250 mm × 10 mm i.d., 5 μm, YMC Corp., Ltd., Kyoto, Japan), with further HPLC purifications performed on a Waters 2695 separation module combined with a Waters 2489 UV detector and an Agilent Zorbax Eclipse Plus C18 column (250 mm × 4.6 mm i.d., 5 μm, Agilent Corp., Palo Alto, CA, USA). GC-MS analyses were conducted on a GCMS-QP2010S (Shimadzu Corp., Kyoto, Japan) with DB-5MS column (i.d. = 0.25 μm, length = 30 m, Agilent Technologies).Plant material
The seeds of C. oleifera were collected in Huangshan, Anhui Province, P. R. China, in October 2012. The plant material was identified by one of the authors (Prof. G.H. Bao), and a voucher specimen (no. 2012-10 Hou) was deposited in State Key Laboratory of Tea Plant Biology and Utilization, Anhui Agricultural University.Extraction and isolation
The tea seeds (2.0 kg) were crushed into powder and extracted three times with 70% EtOH (3 × 10 L) at 60 °C under reflux for 4 h each time. The extract was subjected to reduced pressure evaporation to obtain EtOH concentrated solution (350 g), which was suspended in H2O and extracted successively with petroleum ether, EtOAc and n-BuOH. The n-BuOH fraction (80 g) was subjected to CC on silica gel and eluted with a gradient of EtOAc–MeOH (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 90
0, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 70
20, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 60
30, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 50
40, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 40
50, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, 30
60, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, 20
70, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, 10
80, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, 0
90, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, each 8.0 L). Each fraction was collected, yielding eight major fractions (A–H), and analyzed by TLC. Fraction G (6.5 g) was submitted to CC on Sephadex LH-20 in MeOH to remove the pigments and flavones, yielding four major fractions (I–IV). Fraction II (2.0 g) was subjected to semipreparative HPLC [YMC-Pack ODS-A, CH3CN-0.5% aqueous HCOOH (40
100, each 8.0 L). Each fraction was collected, yielding eight major fractions (A–H), and analyzed by TLC. Fraction G (6.5 g) was submitted to CC on Sephadex LH-20 in MeOH to remove the pigments and flavones, yielding four major fractions (I–IV). Fraction II (2.0 g) was subjected to semipreparative HPLC [YMC-Pack ODS-A, CH3CN-0.5% aqueous HCOOH (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, v/v), 2 mL min−1] to yield five fractions [Fr. 1 (0.83 g), Fr. 2 (0.12 g), Fr. 3 (0.06 g), Fr. 4 (0.11 g), and Fr. 5 (0.55 g)]. Fraction 4 was further purified by HPLC [Agilent C18, CH3CN-0.5% aqueous HCOOH (44
60, v/v), 2 mL min−1] to yield five fractions [Fr. 1 (0.83 g), Fr. 2 (0.12 g), Fr. 3 (0.06 g), Fr. 4 (0.11 g), and Fr. 5 (0.55 g)]. Fraction 4 was further purified by HPLC [Agilent C18, CH3CN-0.5% aqueous HCOOH (44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56, v/v), 1 mL min−1] to afford oleiferasaponin C6 (6.7 mg).
56, v/v), 1 mL min−1] to afford oleiferasaponin C6 (6.7 mg).
OSC6: white amorphous powder; UV (MeOH) λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 208 (3.98), 254 (4.17), 279 (4.32); IR (KBr) νmax (cm−1): 3416, 2950, 2927, 1716, 1638, 1450, 1384, 1309, 1164, 1078, 1046; 1H NMR (pyridine-d5, 600 MHz) and 13C NMR (pyridine-d5, 150 MHz) spectroscopic data, was shown in Table 1, Fig. S3 and S4;† HR-ESI-MS (positive ion mode): m/z 1345.5824 [M + Na]+ (calcd for C65H94O28 Na, 1345.5829), the data were shown in Fig. S2.†
ε): 208 (3.98), 254 (4.17), 279 (4.32); IR (KBr) νmax (cm−1): 3416, 2950, 2927, 1716, 1638, 1450, 1384, 1309, 1164, 1078, 1046; 1H NMR (pyridine-d5, 600 MHz) and 13C NMR (pyridine-d5, 150 MHz) spectroscopic data, was shown in Table 1, Fig. S3 and S4;† HR-ESI-MS (positive ion mode): m/z 1345.5824 [M + Na]+ (calcd for C65H94O28 Na, 1345.5829), the data were shown in Fig. S2.†
| Position | δC | δH | Position | δC | δH |
|---|---|---|---|---|---|
| a 1H (δ ppm, J Hz, s: singlet; d: doublet; brs: broad singlet; m: multiplet). Ac: acetyl; Cin: cinnamoyl; GlcA: glucuronic acid; Gal: galactose; Xyl: xylose. | |||||
| 1 | 38.0 | 0.89m, 1.41m | 22-O-Cin | ||
| 2 | 25.1 | 1.84m, 2.07m | Cin-1 | 167.1 | |
| 3 | 84.5 | 4.01m | Cin-2 | 119.2 | 6.57d (15.6) |
| 4 | 55.0 | Cin-3 | 144.6 | 7.93d (16.2) | |
| 5 | 48.3 | 1.39m | 1′ | 134.8 | |
| 6 | 20.3 | 0.95m, 1.35m | 2′, 6′ | 128.3 | 7.31m |
| 7 | 32.3 | 1.14m, 1.50m | 3′, 5′ | 129.1 | 7.29m |
| 8 | 40.2 | 4′ | 130.5 | 7.30m | |
| 9 | 46.6 | 1.75m | 3-O- | ||
| 10 | 35.9 | GlcA-1′ | 104.0 | 4.75d (7.2) | |
| 11 | 23.7 | 1.76m, 1.85m | GlcA-2′ | 77.8 | 4.48m |
| 12 | 123.1 | 5.39brs | GlcA-3′ | 84.6 | 4.27m |
| 13 | 142.8 | GlcA-4′ | 69.5 | 4.49m | |
| 14 | 41.6 | GlcA-5′ | 77.0 | 4.10m | |
| 15 | 34.5 | 1.59m, 1.81m | GlcA-6′ | 169.9 | |
| 16 | 68.1 | 4.52brs | COOMe | 52.1 | 3.69s |
| 17 | 48.2 | Gal-1′′ | 102.9 | 5.87d (7.8) | |
| 18 | 39.9 | 3.13m | Gal-2′′ | 73.6 | 4.47m |
| 19 | 47.1 | 1.42m, 3.09m | Gal-3′′ | 75.3 | 4.39m |
| 20 | 36.2 | Gal-4′′ | 70.4 | 4.31m | |
| 21 | 79.4 | 6.64d (10.2) | Gal-5′′ | 76.4 | 4.48m |
| 22 | 74.2 | 6.40d (10.2) | Gal-6′′ | 62.2 | 4.50 (2H, m) |
| 23 | 210.1 | 9.93s | Gal-1′′′ | 101.6 | 5.68d (7.2) |
| 24 | 11.0 | 1.45s | Gal-2′′′ | 83.9 | 4.52m |
| 25 | 15.7 | 0.79s | Gal-3′′′ | 75.0 | 4.29m |
| 26 | 16.7 | 0.80s | Gal-4′′′ | 70.4 | 4.51m |
| 27 | 27.3 | 1.78s | Gal-5′′′ | 76.2 | 4.31m |
| 28 | 63.3 | 3.42m, 3.63m | Gal-6′′′ | 61.6 | 4.34 (2H, m) |
| 29 | 29.3 | 1.09s | Xyl-1′′′′ | 107.7 | 5.03d (7.8) |
| 30 | 20.0 | 1.33s | Xyl-2′′′′ | 76.3 | 4.21m |
| 21-O-Ac | Xyl-3′′′′ | 78.2 | 4.05m | ||
| Ac-1 | 170.9 | Xyl-4′′′′ | 70.7 | 4.33m | |
| Ac-2 | 20.1 | 2.08s | Xyl-5′′′′ | 67.6 | 3.48m, 4.43m |
Acid hydrolysis and GC-MS analysis of the sugar moieties in OSC6
The configuration of sugar units was established after hydrolysis of OSC6 with 1 M HCl, pyridine containing L-cysteine methyl ester hydrochloride, trimethylsilylimidazole and determination of the retention times by GC-MS operating under the experimental conditions previously reported by Zong et al.10 The standard sugar samples were subjected to the same reaction and GC-MS conditions. The sugar units of OSC6 were identified by comparison with authentic samples: D-xylose (tR 16.72 min), D-galactose (tR 22.30 min), D-glucuronic acid methyl ester (tR 23.34 min). D-Xylose, D-galactose and D-glucuronic acid methyl ester were identified in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for OSC6.
1 for OSC6.
Cell culture
Human hepatocellular carcinoma BEL-7402, human gastric carcinoma BGC-823, human breast cancer MCF-7, human promyelocytic leukemia HL-60 and human oral epidermoid carcinoma KB cell lines were obtained from KeyGEN BioTECH (Nanjing, China). Cells were cultured in RPMI-1640 complete medium supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin (100 U mL−1 penicillin and 100 μg mL−1 streptomycin) and 2 mM L-glutamine at 37 °C in a 5% CO2 humidified atmosphere.Cell viability assay
Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as previously described Zong et al.11 Briefly, cell lines in culture medium were placed in a cell of a 96-well plate at a concentration of 4 × 103 cells per mL and incubated at 37 °C in 5% CO2 for 24 h. After 24 h, an additional 100 μL of complete medium with either: no additions (negative control), 0.1% DMSO (solvent control), 10 μg mL−1 Taxol (positive control), or different concentrations of OSC6 ranging from 0.39 μM to 50 μM were added, and incubated for 72 h. Then, 20 μL of MTT solution (5 mg mL−1) were added to the culture medium, and incubated for 4 h. Next, the medium was removed, and the formazan was dissolved with 150 μL DMSO. Absorbance values were measured at 490 nm using an enzyme-linked immunosorbent Reader (EL-x800, BioTek Instruments, Winooski, VT, USA). The IC50 value was the concentration of OSC6 that resulted in 50% inhibition of cell growth, which was graphically calculated as a comparison with control growth.Cell cycle analysis
HL-60 and BEL-7402 cells were seeded in 6-well plates at 5 × 105 cells per well, and incubated for 24 h until adherent. Next, HL-60 and BEL-7402 cells were treated with various concentrations of OSC6 for 72 h, respectively. After treatment with OSC6, the cells were harvested, washed, suspended in ice-cold PBS, and fixed in 70% ethanol at 4 °C overnight. The cells were stained for total DNA content with RNase A and propidium iodide (PI) staining buffer according to the manufacturer's instructions (Becton Dickinson). Cell cycle distribution was analyzed using a flow cytometer (Becton Dickinson, San Jose, CA, USA) and ModFit software V3.0 (Verity Software House, Topsham, ME, USA).Cell apoptosis analysis
Cell apoptosis was assessed using the Annexin V-APC/7-AAD double-staining apoptosis detection kit (Becton Dickinson, San Jose, CA, USA). In brief, HL-60 and BEL-7402 cells were treated with DMSO or OSC6 for 24 h. Next, cells were washed with ice-cold PBS, and incubated for 15 min at room temperature in the dark. The cells were collected and stained according to the manufacturer's instructions. The apoptosis data acquisition and analysis was performed by a FACS Calibur flow cytometer. Basal apoptosis were determined on negative control cells.Western blot analysis
HL-60 and BEL-7402 cells (2 × 105 cells per well) were seeded in 6-well plates. After 24 h, the medium was replaced with fresh culture medium containing different concentrations of OSC6 or DMSO for 72 h. Then cultured cells were lysed in ice-cold RIPA buffer supplemented with protease and phosphatase inhibitors (Pierce, Rockford, IL, USA). Samples were maintained on ice for 30 min and then centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min, and the supernatants were collected and quantified. Total protein concentration was quantified using BCA protein assay. Equal amount of samples were subjected to 12% SDS-PAGE, transferred to nitrocellulose membranes, which was blocked for 1 h at room temperature using 5% non-fat dry milk dissolved in PBS containing 0.1% Tween-20 (PBS-T). The membranes were washed 3 times with PBS-T, and then incubated overnight with the primary antibodies for one of the following: CDK4, cyclin D1, p21, p-p53, p53, Bax, Bcl-2, caspase-3, caspase-9, cytochrome c, and β-actin (KeyGEN BioTECH). After washing with PBS-T, the membranes were incubated with goat anti-rabbit IgG or with goat anti-mouse IgG HRP-conjugated secondary antibodies for 2 h. The immunoreactive bands were detected using an enhanced chemiluminescence (ECL) detection system (Bio-Rad). The protein bands were analyzed using ChemiDoc XRS+ system equipped with Image Lab software v.4.1 (Bio-Rad).
000 × g for 10 min, and the supernatants were collected and quantified. Total protein concentration was quantified using BCA protein assay. Equal amount of samples were subjected to 12% SDS-PAGE, transferred to nitrocellulose membranes, which was blocked for 1 h at room temperature using 5% non-fat dry milk dissolved in PBS containing 0.1% Tween-20 (PBS-T). The membranes were washed 3 times with PBS-T, and then incubated overnight with the primary antibodies for one of the following: CDK4, cyclin D1, p21, p-p53, p53, Bax, Bcl-2, caspase-3, caspase-9, cytochrome c, and β-actin (KeyGEN BioTECH). After washing with PBS-T, the membranes were incubated with goat anti-rabbit IgG or with goat anti-mouse IgG HRP-conjugated secondary antibodies for 2 h. The immunoreactive bands were detected using an enhanced chemiluminescence (ECL) detection system (Bio-Rad). The protein bands were analyzed using ChemiDoc XRS+ system equipped with Image Lab software v.4.1 (Bio-Rad).
Statistical analysis
All activity data are presented as mean ± SD from at least three independent experiments. Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by the Tukey multiple comparison using GraphPad software (Prism, San Diego, CA, USA). A P value of <0.05 was statistically significant.Results and discussion
Isolation and characterization of the triterpenoid saponin
The n-BuOH fraction obtained from the 70% EtOH extract of defatted C. oleifera seeds was subjected to repeated column chromatography (silica gel, Sephadex LH-20), and HPLC to afford a new oleanane-type saponin (Fig. S9†). The structure was established mainly by NMR experiments and HR-ESI-MS.OSC6 was separated as a white amorphous powder. The molecular formula C65H94O28 was deduced from the HR-ESI-MS [M + Na]+ ion at m/z 1345.5824. The IR spectrum (cm−1) showed the presence of hydroxyl (broad peak around 3416), carbonyl (1716), olefinic (1638), and ether (1078, 1046) functional groups. The 1H and 13C NMR spectroscopic data (Table 1) for triterpenoid aglycone moieties of the compound were similar to those reported for isotheasaponin B2,19 except for the location of a C-23 group. The presence of signals at δC 210.1 and δH 9.93, indicated that the replacement of the 23-CH3 group in isotheasaponin B2 by the 23-CHO group (Fig. 1). In addition, the HMBC (heteronuclear multiple-bond correlation) correlations (Fig. 1) between δH 6.64 (H-21 of the aglycone), 2.08 (H-2 of acetyl group) and δC 170.9 (C-1 of acetyl group), as well as between δH 6.40 (H-22 of the aglycone) and δC 167.1 (C-1 of cinnamoyl group), indicated that the acetyl and cinnamoyl groups were located at C-21 and C-22 of the triterpenoid aglycone, respectively. Furthermore, the NOESY (nuclear Overhauser effect spectroscopy) correlations (Fig. 1) between H-21 at δH 6.64 and H-29 at δH 1.09, as well as those between H-22 at δH 6.40 and H-30 at δH 1.33, suggested that H-21 and H-22 are in α- and β-orientations, which means that the acetyl group at C-21 and the cinnamoyl group at C-22 are β- and α-orientations, separately. Thus, the aglycone of OSC6 was elucidated as 16α-hydroxy-21β-O-acetyl-22α-O-cinnamoyl-23α-aldehyde-28-dihydroxymethylene-olean-12-ene, which was identified to be a new aglycone. In addition, the NMR spectroscopic data of the sugar units (Table 1) were very similar to the sugar units of oleiferoside D.9 Moreover, the location of the glycosidic chain was confirmed by the 1H–1H COSY (correlation spectroscopy), HSQC (heteronuclear single-quantum correlation), HMBC and NOESY experiments (Fig. 1, S5–S8†). The identifications of the sugar units were further confirmed by acid hydrolysis and GC-MS analysis, which revealed one unit of D-glucuronic acid methyl ester (GlcA), two units of D-galactose (Gal) and one unit of D-xylose (Xyl). On the basis of the above analysis, all the proton and carbon signals due to the aglycone and glycosidic portion were unambiguously assigned by 1D- and 2D-NMR experiments. The chemical structure of OSC6 was established to be 16α-hydroxy-21β-O-acetyl-22α-O-cinnamoyl-23α-aldehyde-28-dihydroxymethylene-olean-12-ene-3β-O-[β-D-galactopyranosyl-(1 → 2)]-[β-D-xylopyranosyl-(1 → 2)-β-D-galactopyranosyl-(1 → 3)]-β-D-glucopyranosiduronic acid methyl ester.
 | ||
| Fig. 1 Structure and key HMBC, NOESY correlations of OSC6. (A) Structure of OSC6. (B) Key HMBC and NOESY correlations of OSC6. | ||
OSC6 inhibits the viability of five human tumor cell lines
The effect of the isolated compound on five human cancer cell lines (BEL-7402, BGC-823, MCF-7, HL-60 and KB) was tested using the MTT assay. The saponin OSC6 exhibited potent anti-proliferative activities, with IC50 values of 4.023 μM (BEL-7402), 6.001 μM (BGC-823), 9.016 μM (MCF-7), 1.876 μM (HL-60) and 6.119 μM (KB). OSC6 was especially potent toward HL-60 and BEL-7402 cells (Fig. S10 and Table S1†). The cytotoxicity of OSC6 was also measured in normal liver L-O2 cell line. There was a 5-fold higher in normal liver L-O2 cell (IC50 = 19.973 μM) than liver cancer BEL-7402 cell line.Structure–activity relationships were inferred by comparison of the structures and anti-proliferative activities of the seven types of saponins and total saponins isolated from C. oleifera with known cytotoxic activity.10,11 Among these compounds, OSC6 is the most potent against HL-60 and BEL-7402 cells (Table S2†). The cinnamoyl group at C-22 plays an important role in the anti-proliferative activity of oleanane-type saponins.12,20,21 Furthermore, in comparison with that of oleiferasaponin C3,10 it seems that the cinnamoyl group at position C-22, rather than C-28, and the free hydroxy group at C-28 in OSC6 may also play important roles in cytotoxicity. Thus, the activity of OSC6 depends not only on an isolated structural factor, but also on a combination of properties at both the aglycone and the sugar moieties.22
OSC6 anti-proliferative activities: induction of cancer cell apoptosis
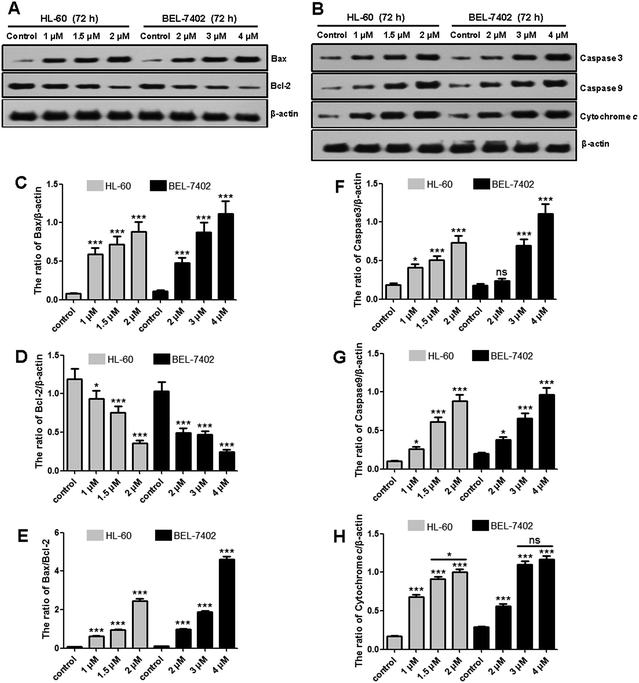
Both HL-60 and BEL-7402 cells were treated with OSC6 followed by staining for apoptosis with Annexin V and APC/7-AAD staining (Fig. 2). OSC6 treatments resulted in an increased number of apoptotic cells. After 72 h of treatment with OSC6 (1 μM and 2 μM), there was an increase in the percentage of apoptotic HL-60 cells (17.27 and 60.72%, respectively) compared with the percentage of apoptotic cells in the negative control (4.87%). The effect on BEL-7402 was similar to that on HL-60 cells.Mitochondrial cytochrome c (Cyto-C) release and caspase activation are important hallmarks of apoptosis.23 When cytosolic Cyto-C meets procaspase-9, the apoptosome is formed, which subsequently stimulates proteolytic activation of caspase-3.24 Protein levels of Cyto-C, caspase-3 and caspase-9 were measured using western blotting (Fig. 3). OSC6 treatment resulted in increased accumulation of Cyto-C and formation of the caspase-3 and caspase-9 active forms (Fig. 3F–H). To further investigate the mechanism underlying OSC6-induced apoptosis, we examined the levels of anti-apoptotic and pro-apoptotic proteins. The action of Bax is counteracted by an anti-apoptotic Bcl-2 family member, such as Bcl-2, which can inhibit mitochondrial pro-apoptotic events.25 Both HL-60 and BEL-7402 cells were treated with OSC6 (0, 1, 1.5 and 2 μM) for 72 h followed by western blotting analysis of protein expression. We observed increased Bax pro-apoptotic protein expression (Fig. 3C) and decreased Bcl-2 anti-apoptotic protein expression (Fig. 3D) following OSC6 treatment. Inhibition of anti-apoptotic proteins of the Bcl-2 family members can also result in activation of the mitochondrial apoptotic signaling pathway.26 Apoptosis is also regulated by the balance between Bcl-2 and Bax proteins. Analysis of signal intensity confirmed that the Bax/Bcl-2 ratio decreased in a dose-dependent manner (Fig. 3E). These results indicated that activation of apoptotic pathways is a mechanism underlying OSC6-induced cell death.
OSC6 arrested cells in the G0/G1 phases of the cell cycle
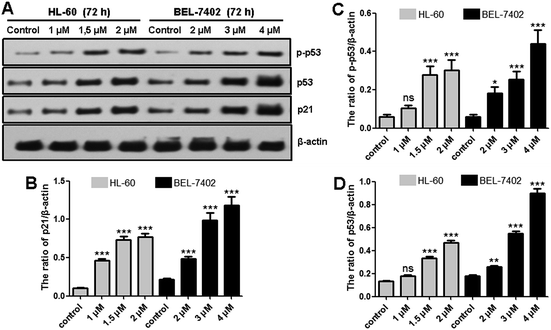
Distribution of the cells between the different phases of the cell cycle was analyzed by flow cytometry of PI stained cells after a 72 h exposure to OSC6. OSC6 clearly perturbed cell cycle progression, inducing an increase in the G0/G1 fraction and a decrease in the S and G2/M fraction (Fig. 4A), reflecting the arrest at G0/G1 in HL-60 cells. Exposure of BEL-7402 cells to OSC6 for 72 h also led to an accumulation of cells in the G0/G1 phase, with a corresponding decrease of cells in the S and G2/M phases. Thus, the inhibition of cell proliferation provoked by a 72 h exposure to OSC6 is mediated by cell cycle arrest in the G0/G1 phase.Cyclin-dependent kinase 4 (CDK4) is a serine/threonine kinase that forms heterodimers with D-type cyclins (cyclin D) and is one of the central promoters of the G1-S transition of the cell cycle.27 We analyzed the expression of the cell cycle promoters CDK4 and cyclin D1 (Fig. 4B–D). Western blot analysis revealed that the OSC6-mediated G0/G1 arrest in HL-60 and BEL-7402 cells was accompanied by the downregulation of CDK4 and cyclin D1. Furthermore, levels of p21 were upregulated after OSC6 treatment (Fig. 5A and B). Cell cycle progression is facilitated by cyclins and CDKs and suppressed by CDK inhibitors p21 and p53. Consistent with the observed G0/G1 cell cycle arrest, exposure to OSC6 diminished the expression of CDK4 and cyclin D1 in both HL-60 and BEL-7402 cells.
OSC6 treatment activates p53 pathway of tumor cell lines
p53 can be activated by many intrinsic and extrinsic factors, and its activation is critical for cell fate determination. Activation of p53 can induce cell apoptosis and bring about cell cycle arrest.28 In addition, p53 regulates numerous downstream molecules, several of which are involved in the death receptor- and mitochondria-mediated apoptotic pathways, which plays a key role in drug-based induction of apoptosis.29,30 Consistent with these findings, we observed a significant p53 accumulation and increase in the population of phosphorylated p53 (p-p53) following OSC6 treatment (Fig. 5). We also detected increased expression of the p53-target protein p21 (Fig. 5). This suggests that p53 plays a vital role in OSC6-induced cell cycle arrest and apoptosis.Conclusions
In summary, the present study reveals that the anti-cancer activity of the novel triterpenoid saponin OSC6 involves activation of the p53 pathway, responsible for inducing apoptosis and G0/G1 cell cycle arrest in cancer cells. OSC6, as a natural product isolated from the C. oleifera seeds, exhibits potent anti-proliferative abilities against human liver, gastric, breast, leukemia and oral epidermoid cancer cells in vitro. An important finding in this study was that OSC6 induced G0/G1 cell cycle arrest that was associated with the up-regulation of p21 and down-regulation of CDK4 and cyclin D1. Moreover, OSC6 treatment induced apoptosis, signaled through a change in the ratio of Bax/Bcl-2, cytochrome c release, caspase-3 and -9 activation, and p53. In the future, efforts should be focused on saponin's anticancer effect in animal models, and its mechanism pathway should be investigated in more detail.Acknowledgements
This research was supported by the projects “Nutrition and Quality & Safety of Agricultural Products, Universities Leading Talent Team of Anhui Province”; The Earmarked Fund for Modern Agro-industry Technology Research System in Tea Industry of Chinese Ministry of Agriculture (nycytx-26) and Natural Science Foundation for Distinguished Young Scholars of Anhui Province (1608085J08).References
- K. I. Block, C. Gyllenhaal, L. Lowe, A. Amedei, A. R. M. R. Amin, A. Amin, K. Aquilano, J. Arbiser, A. Arreola and A. Arzumanyan, in Semin. Cancer Biol., Academic Press, 2015, vol. 35, pp. S276–S304 Search PubMed.
- A. Jemal, F. Bray, M. M. Center, J. Ferlay, E. Ward and D. Forman, Ca-Cancer J. Clin., 2011, 61, 69–90 CrossRef PubMed.
- S. R. Shrivastava, P. S. Shrivastava and J. Ramasamy, Iran. J. Cancer Prev., 2014, 7, 58–59 Search PubMed.
- G. K. Rivera and R. C. Ribeiro, Expert Rev. Hematol., 2014, 7, 649–657 CrossRef CAS PubMed.
- J. M. R. Patlolla and C. V. Rao, Curr. Pharm. Biotechnol., 2012, 13, 147–155 CAS.
- L. Cheng, T. S. Xia, Y. F. Wang, W. B. Zhou, X. Q. Liang, J. Q. Xue, L. Shi, Y. Wang and Q. Ding, PLoS One, 2014, 9, e90848 Search PubMed.
- M. N. Laszczyk, Planta Med., 2009, 75, 1549–1560 CrossRef CAS PubMed.
- T. Rabi and A. Bishayee, Breast Cancer Res. Treat., 2009, 115, 223–239 CrossRef CAS PubMed.
- X. Li, J. P. Zhao, C. P. Peng, Z. Chen, Y. L. Liu, Q. M. Xu, I. A. Khan and S. L. Yang, Planta Med., 2014, 80, 590–598 CrossRef CAS PubMed.
- J. F. Zong, R. L. Wang, G. H. Bao, T. J. Ling, L. Zhang, X. F. Zhang and R. Y. Hou, Fitoterapia, 2015, 104, 7–13 CrossRef CAS PubMed.
- J. F. Zong, Y. R. Peng, G. H. Bao, R. Y. Hou and X. C. Wan, Molecules, 2016, 21, 188 CrossRef PubMed.
- H. Zhou, C. Z. Wang, J. Z. Ye and H. X. Chen, Phytochem. Lett., 2014, 8, 46–51 CrossRef CAS.
- X. F. Zhang, S. L. Yang, Y. Y. Han, L. Zhao, G. L. Lu, T. Xia and L. P. Gao, Molecules, 2014, 19, 7568–7580 CrossRef PubMed.
- M. Feng, Z. Zhu, L. Zuo, L. Chen, Q. Yuan, G. Shan and S. Z. Luo, Anal. Methods, 2015, 7, 5942–5953 RSC.
- P. C. Kuo, T. C. Lin, C. W. Yang, C. L. Lin, G. F. Chen and J. W. Huang, J. Agric. Food Chem., 2010, 58, 8618–8622 CrossRef CAS PubMed.
- J. H. Chen, H. Y. Wu, B. C. Liau, C. M. J. Chang, T. T. Jong and L. C. Wu, Food Chem., 2010, 121, 1246–1254 CrossRef CAS.
- X. F. Zhang, Y. Y. Han, G. H. Bao, T. J. Ling, L. Zhang, L. P. Gao and T. Xia, Molecules, 2012, 17, 11721–11728 CrossRef CAS PubMed.
- L. Y. Chen, J. Chen and H. H. Xu, Fitoterapia, 2013, 84, 123–129 CrossRef CAS PubMed.
- K. Kobayashi, T. Teruya, K. Suenaga, Y. Matsui, H. Masuda and H. Kigoshi, Phytochemistry, 2006, 67, 1385–1389 CrossRef CAS PubMed.
- P. Wang, S. Ownby, Z. Zhang, W. Yuan and S. Li, Bioorg. Med. Chem. Lett., 2010, 20, 2790–2796 CrossRef CAS PubMed.
- K. I. Lee, S. U. Choi and K. R. Lee, Chem. Pharm. Bull., 2012, 60, 1011–1018 CrossRef PubMed.
- L. H. Mu, C. L. Huang, W. B. Zhou, D. H. Guo and P. Liu, Bioorg. Med. Chem. Lett., 2013, 23, 6073–6078 CrossRef CAS PubMed.
- E. M. Creagh, H. Conroy and S. J. Martin, Immunol. Rev., 2003, 193, 10–21 CrossRef CAS PubMed.
- J. E. Chipuk and D. R. Green, Nat. Rev. Mol. Cell Biol., 2005, 6, 268–275 CrossRef CAS PubMed.
- L. Portt, G. Norman, C. Clapp, M. Greenwood and M. T. Greenwood, Biochim. Biophys. Acta, Mol. Cell Res., 2011, 1813, 238–259 CrossRef CAS PubMed.
- E. A. Slee, C. Adrain and S. J. Martin, J. Biol. Chem., 2001, 276, 7320–7326 CrossRef CAS PubMed.
- K. E. Sheppard and G. A. McArthur, Clin. Cancer Res., 2013, 19, 5320–5328 CrossRef CAS PubMed.
- A. J. Levine and M. Oren, Nat. Rev. Cancer, 2009, 9, 749–758 CrossRef CAS PubMed.
- J. K. Sax and W. S. El-Deiry, Cell Death Differ., 2003, 10, 413–417 CrossRef CAS PubMed.
- J. Eliaš, L. Dimitrio, J. Clairambault and R. Natalini, Biochim. Biophys. Acta, Proteins Proteomics, 2014, 1844, 232–247 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: HR-ESR-MS, NMR spectra and anti-proliferative activity data of OSC6. See DOI: 10.1039/c6ra14467e |
| This journal is © The Royal Society of Chemistry 2016 |