Solution and solid-state fluorescence of 2-(2′-hydroxyphenyl)-1,5-benzodiazepin-2-one (HBD) borate complexes†
Hasan Mtiraouia,
Rafik Gharbia,
Moncef Msaddek*a,
Yann Bretonnièreb,
Chantal Andraudb,
Pierre-Yves Renardc and
Cyrille Sabot*c
aUniversité Monastir, Laboratory of Heterocyclic Chemistry Natural Products and Reactivity/CHPNR, Department of Chemistry, Faculty of Science of Monastir, 5000 Monastir, Tunisia
bUniversité Lyon, ENS de Lyon, CNRS, Université Lyon 1, Laboratoire de Chimie, 69342 Lyon, France
cNormandie Univ, CNRS, UNIROUEN, INSA Rouen, COBRA, UMR 6014, 76000 Rouen, France. E-mail: cyrille.sabot@univ-rouen.fr
First published on 5th September 2016
Abstract
A new family of fluorescent 1,5-benzodiazepin-2-one (HBD) borate complexes was prepared in good yields, and fully characterized by means of MS, NMR and IR spectroscopy, as well as X-ray crystal structure analysis for compound 13. Unlike their uncomplexed congeners, most of these cyclic boranils were emissive both in solution and the solid state, with maxima in the range of 426–596 nm. A systematic study of substituent effects revealed that the presence of a halogen atom specifically at position 8 of the fused-aromatic ring system led to an increase in fluorescence intensity in solution while electron rich substituents tended to extinguish the photoluminescence. Finally, a proof-of-concept study highlighted that the amide moiety of the benzodiazepinone framework could be functionalized with a chemical handle useful for subsequent specific modifications.
1. Introduction
Fluorescent boron complexes are an intriguing family of fluorescent small organic molecules that have gained increasing attention in the past two decades due to their high potential for diverse photoluminescence applications including laser dyes, chemosensing1 and biolabeling.2 Furthermore, their charge transport ability was also leveraged in recent and promising applications encompassing dye-sensitized solar cells,3 organic light-emitting diodes, and data storage.4In this context, well-known boron dipyrromethene (BODIPY) dyes have shown remarkable photophysical properties such as sharp absorption and emission spectra, high quantum yields, and excellent chemo- and photostability (not sensitive to their environment in terms of pH or polarity).5 However, these N–B–N bidentate complexes suffer from small Stokes' shifts because of their rigid molecular structures, causing self-quenching and low fluorescence in the solid state. Another drawback may come from their accessibility that occasionally turns out to be tedious. To circumvent these limitations and meet the growing demand for new photoluminescent structures, new generations of aryl-fused6 or bulky7 BODIPY have been reported as well as other families of N–B–N complexes including pyridomethene-,8 formazanate-,9 and aza-pyridyl-isoindoline-containing systems.10 More recently, chelates based on the O–B–O11 and N–B–O12 patterns were also investigated, among which boranils composed of phenoxy-anil ligands have emerged as a promising new family of fluorescent boron complexes (Scheme 1).13
We recently disclosed that 1,5-benzodiazepin-2-ones were emissive in the aggregated and solid-state through the excited-state intramolecular proton transfer (ESIPT) photochemical process.14 In contrast, no fluorescence was observed in organic solvents in which this family of compounds was perfectly soluble. In fact, in solution state, the fluorescence of 1,5-benzodiazepin-2-ones is quenched by intramolecular motions that disrupt the intramolecular hydrogen bonding between the phenolic-OH and the imine nitrogen which was responsible for the ESIPT mechanism. From these results, it could be anticipated that complexation of 1,5-benzodiazepin-2-ones to boron difluoride via a phenoxy-imine chelate, would prevent the free rotation of the phenol ring during the deexcitation process, thus resulting in substantial modifications of their photophysical behaviours in solution. Accordingly, herein is reported the synthesis, and photophysical behaviour of a range of new 2-(2′-hydroxyphenyl)-1,5-benzodiazepin-2-one (HBD) borate complexes.
2. Results and discussion
HBD borate complexes were obtained within three steps from commercially available 4-hydroxycoumarin and 1,2-phenylenediamine derivatives. First, as recently reported,14 the imination/aminolysis sequence between 4-hydroxycoumarins and 1,2-phenylenediamines afforded the desired 2-(2′-hydroxyphenyl)-1,5-benzodiazepin-2-ones as a regioisomeric mixture (Scheme 2). The two isomers, substituted either at position 7 (= R2) or 8 (= R3) of the phenyl ring, were readily separable by column chromatography, except for the methyl and methoxy derivatives, which were isolated as an inseparable mixture, and used as such for the subsequent step. Then, the chemoselective N-methylation or N-propargylation of the amide moiety of 1,5-benzodiazepin-2-ones was achieved by means of NaH and methyl iodide or propargyl bromide in THF, affording the corresponding tertiary amides 2–11 in 74–93% yield. Finally, the boron complexation was achieved by treating benzodiazepin-2-ones with NaH followed by BF3. Et2O, to provide the corresponding complexes 12–22 in excellent 79–94% yield. HBD borate complexes were subsequently fully characterized by means of NMR, IR spectroscopy, and HRMS. First, evidence of the chelation of BF2 by 1,5-benzodiazepin-2-ones was supported by 1H NMR analysis which showed complete disappearance of the downfield proton signal around 14 ppm corresponding to the hydroxyl group. Furthermore, IR spectra revealed strong vibrations in 1000–1200 cm−1 region indicating the presence of B–F bonds, as well as the disappearance of the broad absorption band at 3318 cm−1 characteristic of a hydroxyl vibration. For compound 13, the chelate structure was further confirmed by crystallographic data, which displayed a six-membered ring system containing oxygen–boron–nitrogen linkages (Fig. 1). Crystals for X-ray analysis were obtained by slow evaporation of a DCM/Et2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solution of 13 at room temperature. On the ellipsoid representation (Fig. 1(a)), the fluorine atoms presented a disorder distribution on two crystallographic sites with 73/27% of statistical occupancy. Furthermore, it also appeared that the B1–O2 and B1–N1 bond lengths were 1.43 Å and 1.60 Å respectively, which was in agreement with those reported for analogous six-membered ring BF2 complexes.15 Although the imine moiety was slightly out of the phenol plane, the dihedral angle (−14°) was apparently small enough to enable the delocalization of π-electrons within the system, thus causing fluorescence. An examination of the supramolecular structure of compound 13 revealed the presence of π–π interactions between phenol rings of two adjacent molecules (dashed blue lines), as well as C–H⋯F–B interactions (dashed pink lines), these dimeric interactions playing an important role in the cohesion of the crystal.
3) solution of 13 at room temperature. On the ellipsoid representation (Fig. 1(a)), the fluorine atoms presented a disorder distribution on two crystallographic sites with 73/27% of statistical occupancy. Furthermore, it also appeared that the B1–O2 and B1–N1 bond lengths were 1.43 Å and 1.60 Å respectively, which was in agreement with those reported for analogous six-membered ring BF2 complexes.15 Although the imine moiety was slightly out of the phenol plane, the dihedral angle (−14°) was apparently small enough to enable the delocalization of π-electrons within the system, thus causing fluorescence. An examination of the supramolecular structure of compound 13 revealed the presence of π–π interactions between phenol rings of two adjacent molecules (dashed blue lines), as well as C–H⋯F–B interactions (dashed pink lines), these dimeric interactions playing an important role in the cohesion of the crystal.
Next, the spectroscopic behaviour of this series of compounds was determined, and collected in Table 1. Satisfyingly, unlike their phenol precursors,14 HBD complexes were not only emissive in the solid state but also in solution in organic media such as CHCl3, THF or MeCN, in which they were perfectly soluble (Fig. 2). In fact, as one might expect, stiffening of the 1,5-benzodiazepin-2-one framework upon complexation to boron difluoride prevented intramolecular rotations and vibrations, favouring thus the radiative deexcitation pathway in solution.
| Cmpd | Matrix | λabs (nm) | λem (nm) | Stokes' shift (cm−1) | ΦF |
|---|---|---|---|---|---|
| a Quantum yield determined at 25 °C by the relative method using anthracene (ΦF = 0.26 in EtOH) with excitation at 366 nm.b Absolute quantum yield determined at 25 °C by integrating sphere. | |||||
| 12 | CHCl3a | 370 | 458 | 5192 | 0.10 |
| THFa | 370 | 458 | 5192 | 0.08 | |
| MeCNa | 366 | 458 | 5488 | 0.07 | |
| Solidb | 462 | 0.07 | |||
| 13 | CHCl3 | 373 | 458 | 4975 | 0.14 |
| THF | 369 | 458 | 5266 | 0.15 | |
| MeCN | 365 | 458 | 5563 | 0.09 | |
| Solid | 446 | 0.14 | |||
| 14 | CHCl3 | 373 | 458 | 4975 | 0.10 |
| THF | 368 | 459 | 5387 | 0.13 | |
| MeCN | 364 | 458 | 5638 | 0.11 | |
| Solid | 525 | 0.11 | |||
| 15a/b | CHCl3 | 375 | 458 | 4832 | 0.11 |
| THF | 372 | 456 | 4951 | 0.10 | |
| MeCN | 367 | 456 | 5318 | 0.03 | |
| Solid | 466 | 0.06 | |||
| 16a/b | CHCl3 | 382 | 510 | 6570 | 0.05 |
| THF | 361 | 508 | 8015 | 0.03 | |
| MeCN | 364 | 510 | 7864 | <0.01 | |
| Solid | 571 | 0.30 | |||
| 17a | CHCl3 | 368 | 461 | 5481 | 0.09 |
| THF | 369 | 461 | 5408 | 0.10 | |
| MeCN | 367 | 460 | 5508 | 0.10 | |
| Solid | 520 | <0.01 | |||
| 17b | CHCl3 | 366 | 466 | 5863 | 0.15 |
| THF | 372 | 465 | 5422 | 0.17 | |
| MeCN | 365 | 464 | 5845 | 0.16 | |
| Solid | 564 | 0.04 | |||
| 18a | CHCl3 | 374 | 456 | 5186 | 0.12 |
| THF | 370 | 457 | 5145 | 0.11 | |
| MeCN | 364 | 458 | 5638 | 0.11 | |
| Solid | 527 | 0.08 | |||
| 18b | CHCl3 | 378 | 456 | 4525 | 0.17 |
| THF | 370 | 457 | 5145 | 0.19 | |
| MeCN | 368 | 458 | 5339 | 0.17 | |
| Solid | 508 | 0.08 | |||
| 19a | CHCl3 | 377 | 459 | 4738 | 0.12 |
| THF | 368 | 459 | 5387 | 0.12 | |
| MeCN | 366 | 459 | 5535 | 0.14 | |
| Solid | 566 | 0.14 | |||
| 19b | CHCl3 | 377 | 458 | 4691 | 0.18 |
| THF | 368 | 458 | 5339 | 0.21 | |
| MeCN | 368 | 459 | 5387 | 0.16 | |
| Solid | 496 | 0.09 | |||
| 20a | CHCl3 | 384 | 490 | 5633 | 0.02 |
| THF | 374 | 488 | 6246 | 0.05 | |
| MeCN | 370 | 494 | 6784 | <0.01 | |
| Solid | 596 | 0.05 | |||
| 20b | CHCl3 | 377 | 468 | 5157 | 0.10 |
| THF | 356 | 471 | 6858 | 0.11 | |
| MeCN | 360 | 472 | 6591 | 0.04 | |
| Solid | 583 | 0.01 | |||
| 21 | CHCl3 | 356 | 426 | 4615 | 0.21 |
| THF | 352 | 426 | 4934 | 0.22 | |
| MeCN | 350 | 426 | 5097 | 0.19 | |
| PBS | 350 | 426 | 5097 | 0.10 | |
| Solid | 510 | 0.04 | |||
| 22 | CHCl3 | 362 | 428 | 4259 | 0.23 |
| THF | 363 | 428 | 4183 | 0.24 | |
| MeCN | 360 | 428 | 4413 | 0.20 | |
| PBS | 360 | 430 | 4521 | 0.11 | |
| 23 | CHCl3 | 365 | 468 | 6029 | 0.28 |
| THF | 356 | 471 | 6722 | 0.28 | |
| MeCN | 363 | 472 | 6361 | 0.23 | |
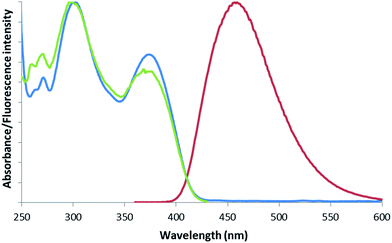 | ||
| Fig. 2 Normalized absorption (blue), emission (red, λex = 350 nm), excitation (green, λem = 458 nm) spectra for compound 2 in CHCl3, at 25 °C. | ||
While absorption maximum wavelengths were slightly affected by the solvent polarity (hypsochromic shift), no noticeable solvatofluorochromism was, however, observed. On the other hand, taken together, this family of compounds exhibited absorption and emission maxima in solution in the range of 350–384 nm and 426–494 nm, respectively, with large Stokes' shifts (4615–6858 cm−1). First, comparing compound 12 with 13, it appeared that N-alkylation of the amide moiety tended to improve the quantum efficiency of the benzodiazepin-2-one derivative both in solution and solid-state. Next, the introduction of a halogen atom such as a chlorine, or a fluorine atom selectively at position 8 on the phenyl moiety of the fused ring system, noticeably increased the quantum yield up to 0.21. In contrast, any modification at position 7 resulted in the decrease of fluorescence intensity. In addition, modification with a nitro substituent caused a ∼30 nm bathochromic shift of the emission wavelength maxima in comparison to the unsubstituted analogue 13, and induced a remarkably large Stokes' shift (up to 6858 cm−1). Regarding solid-state fluorescence of HBD complexes, it is worth noting that large bathochromic shifts were observed from the solution to the solid-state (up to 115 nm for compound 20b). In contrast with results obtained in solution, methoxy substituent increased or decreased the fluorescence intensity depending whether it is positioned on the phenol or on the fused ring system, respectively (0.30 and 0.04 for compounds 16a/b and 21, respectively).
Whilst compound 13 is stable in organic media, somewhat rapid decomplexation of the boron center was however observed in protic solvents such as ethanol or PBS (Fig. 3). In order to circumvent this shortcoming, the increase of the chelating ability of benzodiazepinone ligands toward boron difluoride was investigated by adding an electron-donating methoxy substituent on the phenol ring in para position of the imine moiety (compound 21, Table 1). Gratifyingly, this simple structural modification resulted in a marked increase in stability both in PBS and EtOH, without altering important properties of the benzodiazepin-2-one. Indeed the quantum yields were found to be in the range of 0.19–0.22 in organic solvents and 0.10 in PBS.
Click chemistry16 and related chemical ligation processes17 have become an ubiquitous tool in diverse fields of research for the construction of (bio)molecular systems with a desired profile of properties. Accordingly, the compatibility of HBD complexes with click chemistry was then explored using compound 22, which bears a propargyl functional handle, as reaction partner with benzyl azide. Thus, treatment of 22 with benzyl azide in the presence of CuI (5 mol%) in DCM at room temperature afforded the corresponding triazole derivative 23 in 86% yield. Importantly, subsequent photophysical characterization revealed that quantum yields were high in all solvents tested (0.23–0.28) (Scheme 3).
3. Conclusions
In conclusion, we reported the synthesis of a new family of 15 members of cyclic boranils based on the benzodiazepine scaffold. Subsequent investigation of their optical properties showed that most of them were fluorescent both in solution and solid-state, the vanishing of fluorescence being however specifically observed in solution with the presence of the methoxy substituent on the fused-aromatic ring system. On the other hand, this methoxy group proved highly valuable in terms of hydrolytic stability and quantum yield when positioned on the phenol ring. This promising result suggests that there is room for further improvements so as to use them eventually in bioimaging applications.4. Experimental
All solvents were dried following standard procedures: toluene, methylene chloride (DCM) were obtained from MB SPS-800 apparatus from MBRAUN. THF: distillation over Na°/benzophenone. Cyclohexane and ethyl acetate (EtOAc) were purchased at ACS grade quality and used without further purification. Commercially available reagents were used without further purification, unless otherwise stated. All reactions involving air and moisture sensitive reagents were performed under argon using syringe-septum cap technique. Column chromatography purifications were performed on silica gel (40–63 μm) carried out on Merck DC Kieselgel 60. Thin-layer chromatography (TLC) analyses were carried out on Merck DC Kieselgel 60 F-254 aluminum sheets. The spots were visualized through illumination with UV lamp (λ = 254 nm) and/or staining with KMnO4. Phosphate buffered saline (PBS, 10 mM phosphate + 15 mM NaCl, pH 7.4), aq. 0.1 M and 1.0 M sodium acetate buffer were prepared using water purified with a Milli-Q system (purified to 18.2 MΩ cm).4.1. Instruments and methods
IR spectra were recorded with a universal ATR sampling accessory. 1H and 13C NMR spectra (C13APT or C13CPD experiments) were recorded on a 300 MHz spectrometer. Chemical shifts are expressed in parts per million (ppm) from the residual non-deuterated solvent signal: [D6]DMSO (δH = 2.50, δC = 39.52), multiplicities are described as s (singlet), d (doublet), dd, ddd, etc. (doublet of doublets, doublet of doublets of doublets, etc.), t (triplet), dt (doublet of triplets), td (triplet of doublets), m (multiplet), bs (broad singlet). Coupling constants, J values, are reported in Hz. High-resolution mass spectra (HRMS) were obtained using an orthogonal acceleration time-of-flight (oa-TOF) mass spectrometer equipped with an electrospray source and in the positive and negative modes (ESI±). UV-visible spectra were obtained using a rectangular quartz cell (Open Top, 10 × 10 mm, 3.5 mL). Solid-state fluorescence quantum yields were determined using an integrating sphere instrument (F-3018 Horiba–Jobin–Yvon or G8 GMP) according to a reported method.18 Fluorescence spectroscopic studies in solution were performed with a semi-micro quartz fluorescence cell (Hellma, 104F-QS, light patch base thickness 10 × 4 mm, chamber volume: 1400 μL), with excitation and emission slit widths of 5 nm. Solvents for spectroscopy were spectroscopic grade. Fluorescence quantum yields in 0.1 M PBS pH 7.4 were measured at 25 °C by a relative method using anthracene (ΦF = 0.26 in ethanol)19 as a standard. The following equation was used to determine the relative fluorescence quantum yield in solution:| ΦF(x) = (As/Ax)(Fx/Fs)(nx/ns)2ΦF(s) |
4.2. Syntheses
4-(2-Hydroxyphenyl)-7-methoxy-1,5-benzodiazepin-2-one and 4-(2-hydroxyphenyl)-8-methoxy-1,5-benzodiazepin-2-one. A solution of 4-hydroxycoumarin (1.62 g, 10 mmol) and 4-methoxy-1,2-phenylenediamine (1.08 g, 10 mmol, 1 equiv.) in acetic acid/ethanol (40 mL, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was refluxed for 3 h. Then, the reaction mixture was cooled to room temperature, filtered, and the precipitate washed with methanol several times and purified by chromatography on silica gel as a yellow solid as an inseparable mixture of regioisomers in a 0.09
1, v/v) was refluxed for 3 h. Then, the reaction mixture was cooled to room temperature, filtered, and the precipitate washed with methanol several times and purified by chromatography on silica gel as a yellow solid as an inseparable mixture of regioisomers in a 0.09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (2.05 g, 72% yield) as a yellow solid, mp = 396–298 °C. IR (ATR): νmax: 3150 (NH), 3100 (OH) 1668 (CN), 1595 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.33 (s, 2.25H), 3.81 (s, 3.35H), 6.93–7.02 (m, 4.47H), 7.17 (d, J = 9 Hz, 1.07H), 7.44–7.50 (m, 1.14H), 7.91 (d, J = 9 Hz, 1.07H), 10.55 (s, 1H), 10.66 (s, 0.09H), 14 (s, 1H), 14.18 (s, 0.09H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 38.6, 55.5, 109.9, 115, 117.6, 118.1, 118.9, 123.2, 124.7, 129.7, 137.2, 155.9, 161.4, 163, 165.6 ppm. HRMS (ESI+): calcd for C16H14N2O3 [M + H]+ 282.1083; found 282.1091.
1 ratio (2.05 g, 72% yield) as a yellow solid, mp = 396–298 °C. IR (ATR): νmax: 3150 (NH), 3100 (OH) 1668 (CN), 1595 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.33 (s, 2.25H), 3.81 (s, 3.35H), 6.93–7.02 (m, 4.47H), 7.17 (d, J = 9 Hz, 1.07H), 7.44–7.50 (m, 1.14H), 7.91 (d, J = 9 Hz, 1.07H), 10.55 (s, 1H), 10.66 (s, 0.09H), 14 (s, 1H), 14.18 (s, 0.09H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 38.6, 55.5, 109.9, 115, 117.6, 118.1, 118.9, 123.2, 124.7, 129.7, 137.2, 155.9, 161.4, 163, 165.6 ppm. HRMS (ESI+): calcd for C16H14N2O3 [M + H]+ 282.1083; found 282.1091.
4-(2-Hydroxy-4-methoxyphenyl)-1,5-benzodiazepin-2-one. A solution of 7-methoxy-4-hydroxycoumarin (96 mg, 5 mmol) and 1,2-phenylenediamine (54 mg, 5 mmol, 1 equiv.) in p-xylene (25 mL) was refluxed for 3 h. Then, the reaction mixture was cooled to room temperature, filtered, and the precipitate washed with methanol several times to afford the corresponding compound (1.08 g, 3.82 mmol, 77% yield) as a yellow solid, mp = 288–290 °C. IR (ATR): νmax: 3150 (NH), 3100 (OH) 1673 (CN), 1621 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.56 (s, 2H), 3.81 (s, 3H), 6.51–6.91 (m, 2H), 7.21–7.43 (m, 4H), 7.84 (d, J = 9 Hz, 1H), 10.68 (s, 1H), 14.65 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 38.4, 55.5, 101.4, 106.7, 111.5, 122.1, 124.5, 126.7, 127.1, 131.1, 131.2, 136.2, 162.7, 163.9, 164, 166 ppm. HRMS (ESI+): calcd for C16H15N2O3 [M + H]+ 282.1083; found 282.1091.
General procedure. To a solution of the appropriate 4-phenyl-1,3-dihydro-1,5-benzodiazepin-2-one derivative (1 mmol) in anhydrous THF (20 mL) at 0° C, was added NaH (60% in mineral oil, 0.044 g, 1.1 mmol, 1.1 equiv.). The mixture was stirred for 10 to 15 min before the addition of methyl iodide (0.124 mL, 2 mmol, 2 equiv.). The reaction mixture was maintained at room temperature for 2 h, before removing the solvent under reduced pressure. The crude material was purified by flash column chromatography on silica gel (cyclohexane/EtOAc from 100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 80
0 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain the desired product.
20) to obtain the desired product.
Mixture of 4-(2-hydroxyphenyl)-(1,7)-dimethyl-1,5-benzodiazepin-2-one 4a and 4-(2-hydroxyphenyl)-(1,8)-dimethyl-1,5-benzodiazepin-2-one 4b. (210 mg, 0.75 mmol), 75% yield, isolated as a yellow solid and as an inseparable mixture of regioisomers 4a/4b in a 0.56
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, mp = 80–82 °C. IR (ATR): νmax: 2989 (OH), 2915 (OH), 1667 (CN), 1591 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 2.37 (s, 3H), 2.40 (s, 1.7H), 3.04 (d, J = 12 Hz, 1.29H), 3.29 (s, 3H), 3.31 (s, 1.79H), 4.27 (d, J = 12 Hz, 1.28H), 6.9–7.03 (m, 2.80H), 7.15–7.27 (m, 2.79H), 7.34–7.50 (m, 3.62H), 7.92–7.94 (m, 1.36), 14 (s, 1H), 14.01 (s, 0.39H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 20.1, 20.7, 34.7, 34.8, 37.9, 117.6, 117.6, 117.8, 118.9, 122.2, 122.5, 126.2, 126.4, 128.1, 129.8, 129.8, 133.4, 133.9, 134, 134.8, 135.4, 135.5, 137, 137.4, 161.3, 161.4, 164, 164.5, 165.1, 165.1 ppm. HRMS (ESI+): calcd for C17H16N2O2 [M + H]+ 281.1290; found 281.1280.
1 ratio, mp = 80–82 °C. IR (ATR): νmax: 2989 (OH), 2915 (OH), 1667 (CN), 1591 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 2.37 (s, 3H), 2.40 (s, 1.7H), 3.04 (d, J = 12 Hz, 1.29H), 3.29 (s, 3H), 3.31 (s, 1.79H), 4.27 (d, J = 12 Hz, 1.28H), 6.9–7.03 (m, 2.80H), 7.15–7.27 (m, 2.79H), 7.34–7.50 (m, 3.62H), 7.92–7.94 (m, 1.36), 14 (s, 1H), 14.01 (s, 0.39H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 20.1, 20.7, 34.7, 34.8, 37.9, 117.6, 117.6, 117.8, 118.9, 122.2, 122.5, 126.2, 126.4, 128.1, 129.8, 129.8, 133.4, 133.9, 134, 134.8, 135.4, 135.5, 137, 137.4, 161.3, 161.4, 164, 164.5, 165.1, 165.1 ppm. HRMS (ESI+): calcd for C17H16N2O2 [M + H]+ 281.1290; found 281.1280.
Mixture of 4-(2-hydroxyphenyl)-7-methoxy-1-methyl-1,5-benzodiazepin-2-one 5a and 4-(2-hydroxyphenyl)-8-methoxy-1-methyl-1,5-benzodiazepin-2-one 5b. (263 mg, 0.89 mmol), 89% yield, isolated as a yellow solid and as an inseparable mixture of regioisomers 5a/5b in a 0.09
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, mp = 142–144 °C. IR (ATR): νmax: 3020 (OH), 1657 (CN), 1593 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.06 (d, J = 12 Hz, 1.06H), 3.28 (s, 3H), 3.32 (s, 0.25H), 3.83 (s, 3H), 3.85 (s, 0.25H), 4.27 (d, J = 12 Hz, 1.06H), 6.94–7.08 (m, 4.53H), 7.39–7.59 (m, 2.28H), 7.92–7.95 (m, 1.1H), 13.88 (s, 1H), 14.04 (s, 0.07H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.8, 38, 55.6, 109.4, 114.7, 117.6, 117.8, 119, 123.6, 129.3, 130, 134.1, 138.7, 156.2, 161.3, 164.8, 165 ppm. HRMS (ESI+): calcd for C17H16N2O3 [M]+ 296.1160; found 296.1153.
1 ratio, mp = 142–144 °C. IR (ATR): νmax: 3020 (OH), 1657 (CN), 1593 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.06 (d, J = 12 Hz, 1.06H), 3.28 (s, 3H), 3.32 (s, 0.25H), 3.83 (s, 3H), 3.85 (s, 0.25H), 4.27 (d, J = 12 Hz, 1.06H), 6.94–7.08 (m, 4.53H), 7.39–7.59 (m, 2.28H), 7.92–7.95 (m, 1.1H), 13.88 (s, 1H), 14.04 (s, 0.07H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.8, 38, 55.6, 109.4, 114.7, 117.6, 117.8, 119, 123.6, 129.3, 130, 134.1, 138.7, 156.2, 161.3, 164.8, 165 ppm. HRMS (ESI+): calcd for C17H16N2O3 [M]+ 296.1160; found 296.1153.
4-(2-Hydroxyphenyl)-7-bromo-1-methyl-1,5-benzodiazepin-2-one 6a. (282 mg, 0.82 mmol), 82%, isolated as a yellow solid, mp = 158–160 °C. IR (ATR): νmax: 3010 (OH), 1671 (CN), 1595 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.11 (d, J = 12 Hz, 1H), 3.31 (s, 3H), 4.31 (d, J = 12 Hz, 1H), 6.97–7.02 (m, 2H), 7.40–7.52 (m, 3H), 7.79 (m, 1H), 7.93 (d, J = 9 Hz, 1H), 13.65 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.8, 38.1, 117.6, 117.9, 119.1, 119.2, 125.1, 128.1, 128.5, 130, 134.2, 137, 137.1, 161.2, 165.1, 165.2 ppm. HRMS (ESI+): calcd for C16H13BrN2O2 [M]+ 344.0160; found 344.0169.
4-(2-Hydroxyphenyl)-8-bromo-1-methyl-1,5-benzodiazepin-2-one 6b. (313 mg, 0.91 mmol, 91%), isolated as a yellow solid, mp = 162–164 °C. IR (ATR): νmax: 3000 (OH), 1669 (CN), 1595 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.11 (d, J = 12 Hz, 1H), 3.29 (s, 3H), 4.31 (d, J = 12 Hz, 1H), 6.97–7.02 (m, 2H), 7.45–7.56 (m, 3H), 7.70 (d, J = 3 Hz, 1H), 7.94 (d, J = 9 Hz, 1H), 13.49 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.7, 38.2, 117, 117.7, 117.8, 119.1, 124.5, 128.7, 129.6, 130.1, 134.3, 135.1, 139.2, 161.2, 165.1, 165.7 ppm. HRMS (ESI+): calcd for C16H13BrN2O2 [M]+ 344.0173; found 344.0170.
4-(2-Hydroxyphenyl)-7-chloro-1-methyl-1,5-benzodiazepin-2-one 7a. (252 mg, 0.84 mmol, 84%), isolated as a yellow solid, mp = 170–172 °C. IR (ATR): νmax: 3000 (OH), 1673 (CN), 1590 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.12 (d, J = 12 Hz, 1H), 3.22 (s, 3H), 4.32 (d, J = 12 Hz, 1H), 6.98–7.03 (m, 2H), 7.37–7.52 (m, 3H), 7.69 (d, J = 3 Hz, 1H), 7.93–7.96 (dd, J = 6 Hz, J = 3 Hz, 1H), 13.67 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.8, 36.1, 117.6, 117.8, 119.1, 122.3, 125.2, 127.4, 128.3, 130, 131, 134.2, 136.7, 161.2, 165.1, 165.2 ppm. HRMS (ESI+): calcd for C16H14ClN2O2 [M + H]+ 301.0744; found 301.0742.
4-(2-Hydroxyphenyl)-8-chloro-1-methyl-1,5-benzodiazepin-2-one 7b. (276 mg, 0.92 mmol, 92%), isolated as a yellow solid, mp = 145–147 °C. IR (ATR): νmax: 2980 (OH), 1674 (CN), 1587 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.12 (d, J = 12 Hz, 1H), 3.31 (s, 3H), 4.32 (d, J = 12 Hz, 1H), 7.01 (t, J = 9 Hz, 2H), 7.44–7.61 (m, 4H), 7.93–7.96 (dd, J = 9 Hz, J = 3 Hz, 1H), 13.50 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.8, 38.2, 117.7, 117.8, 119.1, 124.3, 125.8, 126.8, 128.9, 130.1, 134.4, 134.7, 138.9, 161.2, 165.1, 165.7 ppm. HRMS (ESI+): calcd for C16H14ClN2O2 [M + H]+ 301.0744; found 301.0736.
4-(2-Hydroxyphenyl)-7-fluoro-1-methyl-1,5-benzodiazepin-2-one 8a. (230 mg, 0.81 mmol, 81%), isolated as a yellow solid, mp = 188–190 °C. IR (ATR): νmax: 2990 (OH), 1673 (CN), 1594 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.14 (sb, 1H), 3.32 (s, 3H), 4.30 (sb, 1H), 6.97–7.03 (m, 2H), 7.20–7.26 (m, 1H), 7.45–7.56 (m, 3H), 7.92–7.95 (dd, J = 9 Hz, J = 3 Hz, 1H), 13.76 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.7, 37.9, 109, 109.4, 112.6, 112.9, 117.6, 117.8, 119, 128.5, 128.6, 129.9, 134, 134.6, 134.6, 136.8, 136.9, 158.5, 161.2, 161.8, 164.4, 165.1 ppm. HRMS (ESI+): calcd for C16H13FN2O2 [M + H]+ 285.1039; found 285.1031.
4-(2-Hydroxyphenyl)-8-fluoro-1-methyl-1,5-benzodiazepin-2-one 8b. (264 mg, 0.93 mmol, 93%), isolated as a yellow solid, mp = 138–140 °C. IR (ATR): νmax: 2976 (OH), 1667 (CN), 1593 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.11 (d, J = 12 Hz, 1H), 3.20 (s, 3H), 3.31 (d, J = 12 Hz, 1H), 6.98–7.04 (m, 2H), 7.28–7.33 (m, 1H), 7.37–7.41 (dd, J = 9 Hz, J = 3 Hz, 1H), 7.46–7.52 (td, J = 9 Hz, J = 3 Hz, 1H), 7.59–7.64 (m, 1H), 7.93–7.96 (dd, J = 9 Hz, J = 3 Hz, 1H), 13.56 (s, 3H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.9, 38.1, 122.2, 112.5, 114.2, 114.5, 117.6, 117.8, 119.1, 124.4, 124.5, 130.1, 132.4, 132.4, 134.3, 139, 139.1, 156.9, 160.1, 161.2, 165.1, 165.6 ppm. HRMS (ESI+): calcd for C16H13FN2O2 [M + H]+ 285.1039; found 285.1039.
4-(2-Hydroxyphenyl)-7-nitro-1-methyl-1,5-benzodiazepin-2-one 9a. (230 mg, 0.74 mmol, 74%), isolated as a yellow solid, mp = 205–207 °C. IR (ATR): νmax: 2983 (OH), 1674 (CN), 1600 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.20 (d, J = 12 Hz, 1H), 3.40 (s, 3H), 4.41 (d, J = 12 Hz, 1H), 7.03 (t, J = 6 Hz, 2H), 7.50–7.56 (td, J = 9 Hz, J = 3 Hz, 1H), 7.73 (d, J = 9 Hz, 1H), 7.88 (d, J = 6 Hz, 1H), 8.13–8.17 (dd, J = 9 Hz, J = 3 Hz, 1H), 8.37 (d, J = 3 Hz, 1H), 13.29 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.8, 38.6, 117.7, 117.9, 118.4, 119.3, 119.7, 128, 130.4, 134.9, 136, 142.9, 145.1, 161.3, 165.3, 167 ppm. HRMS (ESI+): calcd for C16H14N3O4 [M + H]+ 312.0984; found 312.0979.
4-(2-Hydroxyphenyl)-8-nitro-1-methyl-1,5-benzodiazepin-2-one 9b. (248 mg, 0.80 mmol, 80%), isolated as a yellow solid, mp = 188–190 °C. IR (ATR): νmax: 2983 (OH), 1688 (CN), 1617 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.22 (sb, 1H), 3.40 (s, 3H), 4.41 (sb, 1H), 7.02 (t, J = 6 Hz, 2H), 7.51 (t, J = 6 Hz, 1H), 7.78 (d, J = 9 Hz, 1H), 7.97 (d, J = 6 Hz, 1H), 8.20–8.24 (dd, J = 9 Hz, J = 3 Hz, 1H), 8.33 (d, J = 3 Hz, 1H), 13.23 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 35, 38.5, 117.7, 117.9, 119.2, 121.2, 122.3, 123.8, 130.3, 134.6, 138, 140.9, 143.6, 161.1, 165.2, 166.4 ppm. HRMS (ESI+): calcd for C16H14N3O4 [M + H]+ 312.0984; found 312.0971.
4-(2-Hydroxy-4-methoxyphenyl)-1-methyl-1,5-benzodiazepin-2-one 10. (0.248 g, 0.84 mmol, 84% yield) was obtained as yellow solid, mp = 158–160 °C. IR (ATR): νmax: 3050 (OH) 1658 (CN), 1619 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.02 (d, J = 12 Hz, 1H), 3.33 (s, 3H), 3.81 (s, 3H), 4.21 (d, J = 12 Hz), 6.51–6.60 (m, 2H), 7.29–7.56 (m, 4H), 7.86 (d, J = 9 Hz, 1H), 14.47 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.8, 37.8, 55.5, 101.4, 106.7, 111.3, 122.5, 125.3, 126.4, 126.7, 131.4, 135.7, 137.7, 163.9, 164.1164.3, 165.3 ppm. HRMS (ESI+): calcd for C17H17N2O3 [M + H]+ 297.1239; found 297.1236.
General procedure. To a solution of the appropriate 4-phenyl-1,3-dihydro-1,5-benzodiazepin-2-one derivative (1 mmol) in anhydrous THF (20 mL) at 0° C, was added NaH (60% in mineral oil, 0.044 g, 1.1 mmol, 1.1 equiv.). The mixture was stirred for 10 to 15 min before the addition propargyl bromide (0.122 mL, 1.1 mmol, 1.1 equiv.). The reaction mixture was maintained at 60 °C for 4 h, before removing the solvent under reduced pressure. The crude material was purified by flash column chromatography on silica gel (cyclohexane/EtOAc from 100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 80
0 to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to obtain the desired product.
20) to obtain the desired product.
4-(2-Hydroxyphenyl)-1-(prop-2-yn-1-yl)-1,5-benzodiazepin-2-one 3. (249 mg, 0.86 mmol, 86%) was obtained as a yellow solid, mp = 143–145 °C. IR (ATR): νmax: 3227 (CH), 2983 (OH), 1683 (CN), 1593 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.14 (d, J = 15 Hz, 1H), 3.24 (t, J = 3 Hz, 1H), 3.36 (d, J = 15 Hz, 1H), 4.64–4.67 (dd, J = 6 Hz, J = 3 Hz, 2H), 6.98–7.05 (m, 2H), 7.36–7.51 (m, 4H), 7.74–7.77 (m, 1H), 7.94–7.97 (dd, J = 9 Hz, J = 3 Hz, 1H), 13.86 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 36.4, 37.8, 74.8, 79.5, 117.6, 117.8, 119, 122.2, 125.9, 126.8, 127.3, 130, 133.9, 134.2, 138.2, 161.3, 164.6, 164.8 ppm. HRMS (ESI+): calcd for C18H15N2O2 [M + H]+ 291.1123; found 291.1122.
4-(2-Hydroxy-4-methoxyphenyl)-1-(prop-2-yn-1-yl)-1,5-benzodiazepin-2-one 11. (0.243 g, 0.76 mmol, 76% yield) was obtained as a yellow solid, mp = 148–150 °C. IR (ATR): νmax: 3200 (CH), 3000 (OH), 1685 (CN), 1610 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.09 (d, J = 15 Hz, 1H), 3.23 (t, J = 3 Hz, 1H), 3.82 (s, 3H), 4.27 (d, J = 15 Hz, 1H), 4.62–4.65 (dd, J = 9 Hz, J = 3 Hz, 2H), 6.52 (d, J = 3 Hz, 1H), 6.58–6.62 (dd, J = 9 Hz, J = 3 Hz, 1H), 7.36–7.45 (m, 3H), 7.71–7.74 (m, 1H), 7.87 (d, J = 9 Hz, 1H), 14.40 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 36.4, 37.7, 55.5, 74.8, 79.6, 101.4, 106.8, 111.3, 122.2, 125.9, 126.6, 126.8, 131.5, 133.9, 138.2, 163.9, 164.1, 164.3, 164.7 ppm. HRMS (ESI+): calcd for C19H17N2O3 [M + H]+ 321.1239; found 321.1238.
General procedure. To a solution the appropriate 4-(2′-hydroxyphenyl)-1,5-benzodiazepin-2-one derivative (0.5 mmol) in anhydrous THF (5 mL) at room temperature, was added NaH (60% in mineral oil, 22 mg, 0.55 mmol, 1.1 equiv.). The mixture was stirred for 10 to 15 min before the addition of boron trifluoride diethyl etherate (75 μL, 0.6 mmol, 1.2 equiv.). The reaction mixture was maintained at room temperature for 15–20 min. The crude reaction was filtered through Celite® before removing the solvent under reduced pressure. The crude material was recrystallized from diethyl ether.
4-(2-((Difluoroboranyl)oxy)phenyl)-1,5-benzodiazepin-2-one 12. (125 mg, 0.42 mmol, 83%), isolated as a white solid, mp = 288–290 °C. IR (ATR): νmax: 922 (BN), 1060 (BF), 1120 (BF), 3215 (NH), 1698 (CN), 1651 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.42 (d, J = 15 Hz, 1H), 4.50 (d, J = 15 Hz, 1H), 7.14–7.22 (m, 2H), 7.33–7.42 (m, 2H), 7.53–7.59 (td, J = 6 Hz, J = 3 Hz, 1H), 7.76–7.86 (m, 2H), 8.24–8.27 (dd, J = 6 Hz, J = 3 Hz, 1H), 11.0 (s, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 38.7, 115.4, 119.3, 120.9, 122.6, 124.6, 126.4, 129.7, 131.2, 131.3, 133.9, 138.9, 158.5, 158.6, 165.9, 166.3 ppm. HRMS (ESI−): calcd for C15H10BF2N2O2 [M − H]− 299.0803; found 299.0803.
4-(2-((Difluoroboranyl)oxy)phenyl)-1-methyl-1,5-benzodiazepin-2-one 13. (146 mg, 0.46 mmol, 93%), isolated as a white solid, mp = 233–235 °C. IR (ATR): νmax: 916 (BN), 1070 (BF), 1103 (BF), 1677 (CN), 1599 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.31 (s, 3H), 3.46 (d, J = 12 Hz, 1H), 4.56 (d, J = 12 Hz, 1H), 7.13–7.22 (m, 2H), 7.44–7.49 (td, J = 6 Hz, J = 3 Hz, 1H), 7.61–7.89 (m, 4H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.7, 38.3, 115.2, 119.4, 120.9, 122.8, 125.5, 125.9, 129.9, 131.4, 132.2, 138, 139.1, 158.6, 158.6, 165.3, 167 ppm. HRMS (ESI−): calcd for C16H14BF2N2O2 [M − H]− 313.0970; found 313.0960.
4-(2-((Difluoroboranyl)oxy)phenyl)-1-(prop-2-yn-1-yl)-1,5-benzodiazepin-2-one 14. (145 mg, 0.43 mmol, 86%), isolated as a light green solid, mp = 238–240 °C. IR (ATR): νmax: 918 (BN), 1055 (BF), 1142 (BF), 3249 (CH), 1675 (CN), 1612 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.28 (t, J = 3 Hz, 3H), 3.55 (d, J = 12 Hz, 1H), 4.62 (d, J = 12 Hz, 1H), 4.67 (d, J = 3 Hz, 2H), 7.14–7.24 (m, 2H), 7.48–7.54 (td, J = 9 Hz, J = 3 Hz, 1H), 7.65–7.71 (td, J = 9 Hz, J = 3 Hz, 1H), 7.77–7.89 (m, 3H), 7.26–7.29 (dd, J = 9 Hz, J = 3 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 36.3, 38.2, 75.3, 78.8, 115.2, 119.4, 121, 122.7, 126.1, 129.9, 131.4, 132.8, 136.2, 139.2, 158.6, 158.7, 164.6, 167.1 ppm. HRMS (ESI−): calcd for C18H12BF2N2O2 [M − H]− 337.0960; found 337.0967.
4-(2-((Difluoroboranyl)oxy)phenyl)-(1,7)dimethyl-1,5-benzodiazepin-2-one 15a and 4-(2-((difluoroboranyl)oxy)phenyl)-(1,8)dimethyl-1,5-benzodiazepin-2-one 15b. (142 mg, 0.43 mmol, 87%), isolated as a white solid, and as an inseparable mixture of regioisomers 15a/15b in a 0.56
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, mp = 258–260 °C. IR (ATR): νmax: 970 (BN), 1120 (BF), 1148 (BF), 1602 (CO), 1681 (CN). 1H NMR (300 MHz, [D6]DMSO): δ = 2.42 (s, 3H), 2.45 (s, 0.91H), 3.29 (s, 3H), 3.31 (s, 0.91H), 3.44 (d, J = 12 Hz, 1.3H), 4.55 (d, J = 12 Hz, 1.3H), 7.13–7.30 (m, 2.9H), 7.44–7.64 (m, 3.44H), 7.46–7.81 (m, 1.57H), 8.24–8.27 (dd, J = 9 Hz, J = 3 Hz, 1.3H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 20.5, 20.8, 34.6, 38.3, 115.2, 119.3, 120.9, 122.6, 122.7, 125.6, 126.4, 130.7, 131.3, 131.4, 132, 135, 135.8, 138.9, 139, 140.1, 158.5, 158.6, 165.1, 165.2, 166.7 ppm. HRMS (ESI−): calcd for C17H14BF2N2O2 [M − H] 327.1116; found 327.1123.
1 ratio, mp = 258–260 °C. IR (ATR): νmax: 970 (BN), 1120 (BF), 1148 (BF), 1602 (CO), 1681 (CN). 1H NMR (300 MHz, [D6]DMSO): δ = 2.42 (s, 3H), 2.45 (s, 0.91H), 3.29 (s, 3H), 3.31 (s, 0.91H), 3.44 (d, J = 12 Hz, 1.3H), 4.55 (d, J = 12 Hz, 1.3H), 7.13–7.30 (m, 2.9H), 7.44–7.64 (m, 3.44H), 7.46–7.81 (m, 1.57H), 8.24–8.27 (dd, J = 9 Hz, J = 3 Hz, 1.3H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 20.5, 20.8, 34.6, 38.3, 115.2, 119.3, 120.9, 122.6, 122.7, 125.6, 126.4, 130.7, 131.3, 131.4, 132, 135, 135.8, 138.9, 139, 140.1, 158.5, 158.6, 165.1, 165.2, 166.7 ppm. HRMS (ESI−): calcd for C17H14BF2N2O2 [M − H] 327.1116; found 327.1123.
4-(2-((Difluoroboranyl)oxy)phenyl)-7-methoxy-1-methyl-1,5-benzodiazepin-2-one 16a and 4-(2-((difluoroboranyl)oxy)phenyl)-7-methoxy-1-methyl-1,5-benzodiazepin-2-one 16b. (161 mg, 0.47 mmol, 94%), isolated as a white solid and as an inseparable mixture of regioisomers 16a/16b in a 0.09
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio mp = 208–210 °C. IR (ATR): νmax: 950 (BN), 1051 (BF), 1148 (BF), 1602 (CO), 1680 (CN). 1H NMR (300 MHz, [D6]DMSO): δ = 3.28 (s, 3H), 3.32 (s, 0.27H), 3.46 (d, J = 12 Hz, 1.1H), 3.84 (s, 3H), 3.89 (s, 0.28H), 4.55 (d, J = 12 Hz, 1.09H), 7.13–7.31 (m, 4.49H), 7.6–7.63 (m, 1.05H), 7.79 (t, J = 9 Hz, 1.18H), 8.25 (d, J = 9 Hz, 1.07H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.7, 38.4, 55.7, 109.9, 109.9, 110, 115.1, 116.4, 116.4, 121, 124, 131.4, 131.8, 133, 139.2, 155.8, 158.6, 158.6, 165, 167, 1 ppm. HRMS (ESI+): calcd for C17H15BF2N2O3 [M]+ 344.1143; found 344.1156.
1 ratio mp = 208–210 °C. IR (ATR): νmax: 950 (BN), 1051 (BF), 1148 (BF), 1602 (CO), 1680 (CN). 1H NMR (300 MHz, [D6]DMSO): δ = 3.28 (s, 3H), 3.32 (s, 0.27H), 3.46 (d, J = 12 Hz, 1.1H), 3.84 (s, 3H), 3.89 (s, 0.28H), 4.55 (d, J = 12 Hz, 1.09H), 7.13–7.31 (m, 4.49H), 7.6–7.63 (m, 1.05H), 7.79 (t, J = 9 Hz, 1.18H), 8.25 (d, J = 9 Hz, 1.07H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.7, 38.4, 55.7, 109.9, 109.9, 110, 115.1, 116.4, 116.4, 121, 124, 131.4, 131.8, 133, 139.2, 155.8, 158.6, 158.6, 165, 167, 1 ppm. HRMS (ESI+): calcd for C17H15BF2N2O3 [M]+ 344.1143; found 344.1156.
4-(2-((Difluoroboranyl)oxy)phenyl)-7-bromo-1-methyl-1,5-benzodiazepin-2-one 17a. (176 mg, 0.45 mmol, 90%), isolated as a yellow solid, mp = 260–262 °C. IR (ATR): νmax: 905 (BN), 1052 (BF), 1142 (BF), 1608 (CO), 1689 (CN). 1H NMR (300 MHz, [D6]DMSO): δ = 3.32 (s, 3H), 3.56 (d, J = 12 Hz, 1H), 4.60 (d, J = 12 Hz, 1H), 7.13–7.23 (m, 2H), 7.67–7.93 (m, 4H), 8.27 (d, J = 9 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.8, 38.3, 115.2, 119.4, 121, 122.5, 125.6, 127.6, 128.5, 128.6, 131.5, 139.3, 139.4, 158.6, 158.4, 165.2, 167.3 ppm. HRMS (ESI+): calcd for C16H12BBrF2N2O2 [M]+ 392.0143; found 392.0151.
4-(2-((Difluoroboranyl)oxy)phenyl)-8-bromo-1-methyl-1,5-benzodiazepin-2-one 17b. (186 mg, 0.47 mmol, 95%), isolated as a yellow solid, mp = 163–165 °C. IR (ATR): νmax: 924 (BN), 1053 (BF), 1103 (BF), 1611 (CO), 1698 (CN). 1H NMR (300 MHz, [D6]DMSO): δ = 3.30 (s, 3H), 3.56 (d, J = 12 Hz, 1H), 4.61 (d, J = 12 Hz, 1H), 7.15–7.24 (m, 2H), 7.64–7.94 (m, 4H), 8.29 (d, J = 6 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.7, 38.3, 115, 117, 119.4, 121.1, 125, 128.1, 128.2, 128.2, 131.6, 132.6, 133.2, 137.5, 139.6, 158.7, 158.8, 165, 167.9 ppm. HRMS (ESI+): calcd for C16H12BBrF2N2O2 [M]+ 392.0143; found 392.0136.
4-(2-((Difluoroboranyl)oxy)phenyl)-7-chloro-1-methyl-1,5-benzodiazepin-2-one 18a. (158 mg, 0.45 mmol, 91%), isolated as a yellow solid, mp = 253–255 °C. IR (ATR): νmax: 919 (BN), 1068 (BF), 1100 (BF), 1677 (CN), 1612 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.32 (s, 3H), 3.56 (d, J = 12 Hz, 1H), 4.60 (d, J = 12 Hz, 1H), 7.13–7.23 (m, 2H), 7.54–7.58 (dd, J = 9 Hz, J = 3 Hz, 1H), 7.77–7.86 (m, 3H), 8.26–8.29 (dd, J = 9 Hz, J = 3 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.7, 38.3, 115.1, 119.4, 121, 122.8, 125.6, 127.6, 131.1, 131.5, 134, 139.2, 139.4, 158.6, 158.7, 165.1, 167.3 ppm. HRMS (ESI−): calcd for C16H11BClF2N2O2 [M − H]− 347.0570; found 347.0565.
4-(2-((Difluoroboranyl)oxy)phenyl)-8-chloro-1-methyl-1,5-benzodiazepin-2-one 18b. (161 mg, 0.46 mmol, 93%), isolated as a yellow solid, mp = 238–240 °C. IR (ATR): νmax: 927 (BN), 1053 (BF), 1101 (BF), 1698 (CN), 1612 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.31 (s, 3H), 3.57 (d, J = 12 Hz, 1H), 4.62 (d, J = 12 Hz, 1H), 7.15–7.64 (m, 2H), 7.73–7.83 (m, 4H), 8.28–8.31 (dd, J = 9 Hz, J = 3 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.7, 38.3, 115, 119.4, 121.1, 124.9, 125.3, 129, 129.7, 131.6, 133, 137.2, 139.6, 158.7, 158.8, 165, 167.9 ppm. HRMS (ESI−): calcd for C16H11BClF2N2O2 [M − H]− 347.0570; found 347.0573.
4-(2-((Difluoroboranyl)oxy)phenyl)-7-fluoro-1-methyl-1,5-benzodiazepin-2-one 19a. (146 mg, 0.44 mmol, 88%), isolated as a yellow solid, mp = 258–260 °C. IR (ATR): νmax: 920 (BN), 1059 (BF), 1121 (BF), 1694 (CN), 1592 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.32 (s, 3H), 3.55 (d, J = 12 Hz, 1H), 4.60 (d, J = 12 Hz, 1H), 7.13–7.23 (m, 2H), 7.34–7.41 (m, 1H), 7.60–7.65 (dd, J = 12 Hz, J = 3 Hz, 1H), 7.76–7.91 (m, 2H), 8.26–8.29 (dd, J = 9 Hz, J = 3 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.7, 38.3, 109.6, 110, 112.9, 113.2, 115.1, 119.3, 121, 128, 128.1, 128.9, 131.4, 139.2, 139.6, 139.7, 158.5, 158.6, 159.9, 163.2, 165.2, 166.9 ppm. HRMS (ESI−): calcd for C16H11BF3N2O2 [M − H]− 331.0866; found 331.0907.
4-(2-((Difluoroboranyl)oxy)phenyl)-8-fluoro-1-methyl-1,5-benzodiazepin-2-one 19b. (151 mg, 0.46 mmol, 91%), isolated as a yellow solid, mp = 238–240 °C. IR (ATR): νmax: 916 (BN), 1065 (BF), 1183 (BF), 1677 (CN), 1592 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.31 (s, 3H), 3.54 (d, J = 12 Hz, 1H), 4.60 (d, J = 12 Hz, 1H), 7.14–7.24 (m, 2H), 7.55–7.60 (m, 2H), 7.73–7.81 (m, 2H), 8.27–8.30 (dd, J = 9 Hz, J = 3 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.8, 38.3, 112, 112.4, 115, 117.3, 117.6, 119.4, 121.1, 125.1, 125.2, 131.6, 133, 133.2, 135, 139.6, 156.3, 158.7, 158.7, 159.5, 165.1, 167.9 ppm. HRMS (ESI−): calcd for C16H11BF3N2O2 [M − H]− 331.0866; found 331.0875.
4-(2-((Difluoroboranyl)oxy)phenyl)-7-nitro-1-methyl-1,5-benzodiazepin-2-one 20a. (147 mg, 0.41 mmol, 82%), isolated as a yellow solid, mp = 283–285 °C. IR (ATR): νmax: 987 (BN), 1025 (BF), 1132 (BF), 1692 (CN), 1612 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.40 (s, 3H), 3.60 (d, J = 12 Hz, 1H), 4.68 (d, J = 12 Hz, 1H), 7.16–7.26 (m, 2H), 7.82–7.87 (td, J = 9 Hz, J = 3 Hz, 1H), 8.07–8.10 (dd, J = 9 Hz, J = 3 Hz, 1H), 8.29–8.35 (m, 2H), 8.45 (d, J = 3 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 34.8, 38.4, 115.1, 118.7, 119.5, 119.9, 121.2, 127.7, 127.8, 131.8, 136.8, 138.8, 140.1, 159, 159.1, 165.2, 168.9 ppm. HRMS (ESI−): calcd for C16H11BF2N3O4 [M − H]− 358.0811; found 358.0805.
4-(2-((Difluoroboranyl)oxy)phenyl)-8-nitro-1-methyl-1,5-benzodiazepin-2-one 20b. (141 mg, 0.39 mmol, 79%), isolated as a yellow solid, mp = 295–297 °C. IR (ATR): νmax: 929 (BN), 1049 (BF), 1145 (BF), 1708 (CN), 1618 (CO). 1H NMR (300 MHz, [D6]DMSO): δ = 3.38 (s, 3H), 3.63 (d, J = 12 Hz, 1H), 4.71 (d, J = 12 Hz, 1H), 7.17–7.26 (m, 2H), 7.82–7.87 (td, J = 9 Hz, J = 3 Hz, 1H), 7.92 (d, J = 9 Hz, 1H), 8.32–8.35 (dd, J = 9 Hz, J = 3 Hz, 1H), 8.45–8.48 (dd, J = 9 Hz, J = 3 Hz, 1H), 8.66 (t, J = 3 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 35, 38.4, 115, 119.5, 121.2, 121.7, 121.8, 124.2, 124.5, 131.7, 132.1, 140, 143.2, 158.9, 158.9, 165.1, 166.7 ppm. HRMS (ESI−): calcd for C16H11BF2N3O4 [M − H]− 358.0811; found 358.0810.
4-(2-((Difluoroboranyl)oxy)-4-methoxyphenyl)-1-methyl-1,5-benzodiazepin-2-one 21. (159 mg, 0.46 mmol, 93%), isolated as a yellow solid, mp = 228–230 °C. IR (ATR): νmax: 950 (BN), 1033 (BF), 1148 (BF), 1610 (CO), 1685 (CN). 1H NMR (300 MHz, [D6]DMSO): δ = 3.31 (s, 3H), 3.39 (d, J = 12 Hz, 1H), 3.91 (s, 3H), 4.46 (d, J = 12 Hz, 1H), 6.67–6.69 (m, 2H), 7.43–7.83 (m, 4H), 8.15 (d, J = 9 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 39.9, 43.3, 61.5, 107.3, 114.2, 115.6, 128, 130.7, 131, 134.6, 137.7, 138.4, 143.2, 166.7, 166.7, 170.6, 170.7, 173.7 ppm. HRMS (ESI+): calcd for C17H15BF2N2O3 [M]+ 344.1143; found 344.1147.
4-(2-((Difluoroboranyl)oxy)-4-methoxyphenyl)-1-(prop-2-yn-1-yl)-1,5-benzodiazepin-2-one 22. (158 mg, 0.43 mmol, 86%), isolated as a yellow solid, mp = 160–162 °C. IR (ATR): νmax: 1016 (BN), 1034 (BF), 1149 (BF), 1616 (CO), 1686 (CN). 1H NMR (300 MHz, [D6]DMSO): δ = 3.26 (t, J = 3 Hz, 1H), 3.47 (d, J = 12 Hz, 1H), 3.91 (s, 3H), 4.50 (d, J = 12 Hz, 1H), 4.65 (d, J = 3 Hz, 2H), 6.67–6.81 (m, 2H), 7.45–7.84 (m, 4H), 8.17 (d, J = 9 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 36.3, 38, 56.3, 75.3, 78.9, 102.1, 108.9, 110.4, 122.7, 126, 126.1, 129.3, 133.1, 133.1, 136.2, 161.5, 164.7, 165.4, 168.5 ppm. HRMS (ESI−): calcd for C19H14BF2N2O3 [M − H]− 367.1066; found 367.1072.
1-((1-Benzyl-1H-1,2,3-triazol-4-yl)methyl)-4-(2-((difluoroboranyl)oxy)-4-methoxyphenyl)-1,5-benzodiazepin-2-one 23. to a mixture of compound 22 (184 mg, 0.5 mmol) and (azidomethyl)benzene (80 mg, 0.6 mmol, 1.2 eq.) in 5 mL DCM was added CuI (5 mg, 0.025 mmol, 5 mol%) and Et3N (70 μL, 0.5 mmol, 1 eq.). The reaction mixture was stirred at room temperature for 3 h. The crude reaction was filtered through Celite®, the filtrate was concentrated under reduced pressure. The corresponding cycloadduct was obtained by crystallization in a minimum of Et2O. The desired product (205 mg, 0.82 mmol, 82% yield) was obtained as a white solid, mp = 148–150 °C. IR (ATR): νmax: 1016 (BN), 1052 (BF), 1143 (BF), 1617 (CO), 1687 (CN). 1H NMR (300 MHz, [D6]DMSO): δ = 3.45 (d, J = 12 Hz, 1H), 3.91 (s, 3H), 4.48 (d, J = 12 Hz, 1H), 5.07 (s, 2H), 5.47 (d, J = 18 Hz, 1H), 5.53 (d, J = 18 Hz, 1H), 6.69–6.80 (m, 2H), 7.17–7.35 (m, 5H), 7.34 (m, 1H) 7.59 (m, 1H), 7.67 (s, 1H(triazole)), 7.81 (d, J = 6 Hz, 1H), 7.94 (d, J = 6 Hz, 1H), 8.15 (d, J = 9 Hz, 1H) ppm. 13C NMR (75 MHz, [D6]DMSO): δ = 42.9, 52.8, 56.3, 67, 102.1, 109, 110.4, 123.5, 126, 127.7, 128, 128.7, 129.3, 133.2, 135.8, 136.7, 142.8, 161.5, 161.6, 164.6, 165.6, 168.5 ppm. HRMS (ESI−): calcd for C26H21BF2N5O3 [M − H]− 500.1734; found 500.1712.
Acknowledgements
This work was also partially supported by Rouen University, INSA Rouen, Rouen University, the Centre National de la Recherche Scientifique (CNRS), Region Haute-Normandie (CRUNCh network), and the Labex SynOrg (ANR-11-LABX-0029). The authors are grateful to Mrs Amna Benzarti and Miss Nadia Msaddek, NMR service at the Faculty of Monastir, University of Monastir for the NMR analysis and to the Ministry of Higher Education and Scientific Research of Tunisia for financial support (LR11ES39). The authors also thank Albert Marcual (CNRS) for HRMS analyses, Patricia Martel (University of Rouen) for IR analyses, Dr Morgane Sanselme (University of Rouen) for X-ray diffraction analyses.Notes and references
- S. Atilgan, T. Ozdemir and E. U. Akkaya, Org. Lett., 2008, 10, 4065–4067 CrossRef CAS PubMed.
- L. Wu, A. Loudet, R. Barhoumi, R. C. Burghardt and K. Burgess, J. Am. Chem. Soc., 2009, 131, 9156–9157 CrossRef CAS PubMed.
- T. Rousseau, A. Cravino, E. Ripaud, P. Leriche, S. Rihn, A. De Nicola, R. Ziessel and J. Roncali, Chem. Commun., 2010, 46, 5082–5084 RSC.
- H.-R. Zheng, L.-Y. Niu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tunga and Q.-Z. Yang, RSC Adv., 2016, 6, 41002–41006 RSC.
- For reviews on BODYPI dyes, see: (a) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed; (b) R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496–501 RSC; (c) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed, 2008, 47, 1184–1201 CrossRef CAS PubMed.
- C. Yu, Q. Wu, J. Wang, Y. Wei, E. Hao and L. Jiao, J. Org. Chem., 2016, 81, 3761–3770 CrossRef CAS PubMed.
- K. Benelhadj, J. Massue, P. Retailleau, S. Chibani, B. Le Guennic, D. Jacquemin, R. Ziessel and G. Ulrich, Eur. J. Org. Chem., 2014, 7156–7164 CrossRef CAS and references cited therein.
- Y. Kubota, T. Tsuzuki, K. Funabiki, M. Ebihara and M. Matsui, Org. Lett., 2010, 12, 4010–4013 CrossRef CAS PubMed.
- S. M. Barbon, V. N. Staroverov and J. B. Gilroy, J. Org. Chem., 2015, 80, 5226–5235 CrossRef CAS PubMed.
- Y. Wu, H. Lu, S. Wang, Z. Li and Z. Shen, J. Mater. Chem. C, 2015, 3, 12281–12289 RSC.
- (a) V. B. Kovalska, K. D. Volkova, A. V. Manaev, M. Y. Losytskyy, I. N. Okhrimenko, V. F. Traven and S. M. Yarmoluk, Dyes Pigm., 2010, 84, 159–164 CrossRef CAS; (b) K. Kamada, T. Namikawa, S. Senatore, C. Matthews, P.-F. Lenne, O. Maury, C. Andraud, M. Ponce-Vargas, B. Le Guennic, D. Jacquemin, P. Agbo, D. D. An, S. S. Gauny, X. Liu, R. J. Abergel, F. Fages and A. D’Aléo, Chem.–Eur. J., 2016, 22, 5219–5232 CrossRef CAS PubMed.
- (a) H.-J. Son, W.-S. Han, K.-R. Wee, J.-Y. Chun, K.-B. Choi, S. J. Han, S.-N. Kwon, J. Ko, C. Lee and S. O. Kang, Eur. J. Inorg. Chem., 2009, 1503–1513 CrossRef CAS; (b) A. Zakrzewska, E. Kolehmainen, A. Valkonen, E. Haapaniemi, K. Rissanen, L. Chęcińska and B. Ośmiałowski, J. Phys. Chem. A, 2013, 117, 252–256 CrossRef CAS PubMed; (c) H. S. Kumbhar and G. S. Shankarling, Dyes Pigm., 2015, 122, 85–93 CrossRef CAS; (d) K. Benelhadj, J. Massue and G. Ulrich, New J. Chem., 2016, 40, 5877–5884 RSC.
- (a) D. Frath, S. Azizi, G. Ulrich, P. Retailleau and R. Ziessel, Org. Lett., 2011, 13, 3414–3417 CrossRef CAS PubMed; (b) D. Frath, S. Azizi, G. Ulrich and R. Ziessel, Org. Lett., 2012, 14, 4774–4777 CrossRef CAS PubMed; (c) S. Chibani, A. Charaf-Eddin, B. Le Guennic and D. Jacquemin, J. Chem. Theory Comput., 2013, 9, 3127–3135 CrossRef CAS PubMed; (d) K. Dhanunjayarao, V. Mukundam, M. Ramesh and K. Venkatasubbaiah, Eur. J. Inorg. Chem., 2014, 539–545 CrossRef CAS; (e) F. Cardona, J. Rocha, A. M. S. Silva and S. Guieu, Dyes Pigm., 2014, 111, 16–20 CrossRef CAS; (f) J. Dobkowski, P. Wnuk, J. Buczyńska, M. Pszona, G. Orzanowska, D. Frath, G. Ulrich, J. Massue, S. Mosquera-Vázquez, E. Vauthey, C. Radzewicz, R. Ziessel and J. Waluk, Chem.–Eur. J., 2015, 21, 1312–1327 CrossRef CAS PubMed; (g) G. Wesela-Bauman, M. Urban, S. Luliński, J. Serwatowski and K. Woźniak, Org. Biomol. Chem., 2015, 13, 3268–3279 RSC; (h) T. Tomakinian, R. Guillot, C. Kouklovsky and G. Vincent, Chem. Commun., 2016, 52, 5443–5446 RSC.
- H. Mtiraoui, R. Gharbi, M. Msaddek, Y. Bretonnière, C. Andraud, C. Sabot and P.-Y. Renard, J. Org. Chem., 2016, 81, 4720–4727 CrossRef CAS PubMed.
- (a) Y. Kubota, H. Hara, S. Tanaka, K. Funabiki and M. Matsui, Org. Lett., 2011, 13, 6544–6547 CrossRef CAS PubMed; (b) Y. Kubota, Y. Ozaki, K. Funabiki and M. Matsui, J. Org. Chem., 2013, 78, 7058–7067 CrossRef CAS PubMed.
- (a) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS; (b) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905–4979 CrossRef CAS PubMed.
- Recent examples of ligation tools for the construction of (bio)molecular systems: (a) K. Hoogewijs, D. Buyst, J. M. Winne, J. C. Martins and A. Madder, Chem. Commun., 2013, 49, 2927–2929 RSC; (b) L.-A. Jouanno, A. Chevalier, N. Sekkat, N. Perzo, H. Castel, A. Romieu, N. Lange, C. Sabot and P.-Y. Renard, J. Org. Chem., 2014, 79, 10353–10366 CrossRef CAS PubMed; (c) S. Hörner, C. Uth, O. Avrutina, H. Frauendorf, M. Wiessler and H. Kolmar, Chem. Commun., 2015, 51, 11130–11133 RSC; (d) A. Grassin, M. Claron and D. Boturyn, Chem.–Eur. J., 2015, 21, 6022–6026 CrossRef CAS PubMed; (e) S. M. Agten, D. P. L. Suylen and T. M. Hackeng, Bioconjugate Chem., 2016, 27, 42–46 CrossRef CAS PubMed; (f) B. Akgun and D. G. Hall, Angew. Chem., Int. Ed, 2016, 55, 3909–3913 CrossRef CAS PubMed.
- (a) M. Ipuy, Y.-Y. Liao, E. Jeanneau, P. L. Baldeck, Y. Bretonnière and C. Andraud, J. Mater. Chem. C, 2016, 4, 766–779 RSC; (b) J. C. de Mello, H. F. Wittmann and R. H. Friend, Adv. Mater., 1997, 9, 230 CrossRef CAS; (c) L. Porrès, A. Holland, L.-O. PÅlsson, A. P. Monkman, C. Kemp and A. Beeby, J. Fluoresc., 2006, 16, 267 CrossRef PubMed.
- A. M. Brouwer, Pure Appl. Chem., 2011, 83, 2213–2228 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1490358. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra19246g |
| This journal is © The Royal Society of Chemistry 2016 |





