Rod-like micelles of octadecyltrimethylammonium bromide and their freezing upon solubilised styrene polymerisation†
Jianxi Zhao*,
Hongbin Yu and
Shenglu Deng
Institute of Colloid and Interface Chemistry, College of Chemistry and Chemical Engineering, Fuzhou University, Fuzhou, Fujian 350108, P. R. China. E-mail: jxzhao.colloid@fzu.edu.cn
First published on 20th September 2016
Abstract
The association of octadecyltrimethylammonium bromide (C18TABr) in ethanol/water (10/90 wt%) solvent has been studied using light-scattering measurements. With the help of sodium 2-naphthalenesulfonate (SNphs), C18TABr formed rod-like micelles. Styrene was solubilised into the templating C18TABr micelles with a mole ratio of SNphs to surfactant (nSNphs/nC18TABr) of 0.5, which was examined by dynamic light-scattering (DLS) and UV-spectral measurements. Then the solubilised styrene was polymerised in situ. Dilution experiments were performed to test the freezing effect. It was found that the amount of solubilising styrene, represented as the mole ratio of styrene to surfactant (nst/nsurf), should be greater than 2.7 so as to achieve a good freezing effect. In the case of nst/nsurf = 4.4, well-frozen rod-like micelles were obtained and their morphologies were evinced by TEM images. These frozen micelles were stable even in the 400% dilution test.
Introduction
Amphiphilic surfactants can self-assemble into a variety of aggregate structures such as spherical micelles, rod-like or worm-like micelles, vesicles, or lamellar phases in polar or non-polar solvents.1,2 These organised surfactant aggregates are extensively used in a wide range of applications. However, because surfactant self-aggregation is controlled by thermodynamics,2,3 changes in concentration, temperature, salinity, pH, etc., modify their microstructures. It is therefore of significance to freeze these microstructures for aggregate applications. Two approaches have been tried: one was to polymerise the monomers, such as styrene, that were previously solubilised in the aggregates, so as to fix the micellar microstructures,4–8 another was to use polymerisable surfactants.9–11 The former approach was convenient, and more common than the latter.In the present work, we were interested in the approach of monomer polymerisation. Here a key problem is how to ensure that the aggregate structure is not destroyed during styrene solubilisation and the ensuing polymerisation. Moreover, it is more difficult to ensure the morphology of unsymmetrical aggregates, such as short rods, than that of symmetrical aggregates such as spherical micelles or circular vesicles.
Rod-like micelles can be formed by cetyltrimethylammonium bromide/chloride (C16TABr or C16TACl) with the help of simple salts12–15 or hydrotropes.16–22 Hydrotropes, in which sodium salicylate (NaSal) was used the most frequently, were far more effective than simple salts for this purpose. However, NaSal generally favours the induction of rapid growth of the initial rods into thread-like micelles due to its strong interactions with the surfactant. For certain applications, for example in colloidal crystals, short rods are required when using the frozen aggregates as new colloidal units to obtain ordered periodic arrays.23 Unfortunately, the aim of only freezing rod-like micelles has not yet been realised using the method of styrene solubilisation and polymerisation.
In this issue, we reported a study on freezing rod-like micelles according to the first approach described above. For this purpose, a cationic surfactant, octadecyltrimethylammonium bromide (C18TABr), and a hydrotrope, sodium 2-naphthalenesulfonate (SNphs), were chosen, by which we obtained rod-like micelles: we then solubilised and polymerised styrene monomers, by which the frozen rod-like micelles were then obtained.
Results and discussion
Aggregation of C18TABr in the presence of SNphs
Dynamic light-scattering was used to characterise the aggregation of C18TABr in ethanol/water (10/90 wt%) in the presence of SNphs. The time correlation functions of scattering intensity were measured at a 90° angle for the samples of C18TABr (15 mmol L−1) with different mole ratios of SNphs to C18TABr, nSNphs/nC18TABr. The experimental data were analysed by the CONTIN program to obtain the intensity-fraction distributions around each characteristic aggregate size (Fig. S1, ESI†).25,26 For the sample of C18TABr alone (without SNphs), only a single intensity-fraction distribution was observed around a large aggregate size (the apparent hydrodynamic radius Rh) of c. 100 nm. With the addition of SNphs, bimodal intensity-distributions appeared at nSNphs/nC18TABr ≥ 0.1, besides the original large aggregate size, a new characteristic size of c. 2 nm was detected (Fig. S1, ESI†).Upon further increasing nSNphs/nC18TABr, the large aggregates disappeared at nSNphs/nC18TABr > 0.35, while the small particles, after remaining unchanged until nSNphs/nC18TABr < 0.47, started to grow. Fig. 1 shows this SNphs effect, where Rh is shown as a function of nSNphs/nC18TABr.
To understand the behaviour of C18TABr without, and with, SNphs, it is necessary to review the aggregation of long-tailed quaternary ammonium surfactants such as C22TABr, C20TABr, or C18TABr in ethanol/water solvent. As reported in our previous issue,28 these surfactants, which were dissolved in ethanol in advance, can self-assemble to form micelles, upon the addition of water to a certain extent. However, solvent ethanol was found to swell the micellar cores, which gave rise to gaps between the long-tail surfactant molecules within the micelle, and exposed the alkyl tails to the polar solvent. This resulted in secondary association of the micelle into large aggregates.28 This was only the case for C18TABr without SNphs (C18TABr alone) as shown in Fig. S1† where large aggregates were observed.
The addition of salt can screen the charges of the headgroups of such surfactants and promote the closer packing of these molecules in the micelles. Thereby, secondary aggregation decreased and even disappeared with addition of salt as seen in Fig. 1. The characteristic parameter, βLD, indicated the critical molar ratio of SNphs after which the large aggregates disappeared. Another parameter was βC that indicated the critical point after which the core–shell micelles began to grow. The values of both are listed in Table 1.
| SBzs | SNphs | NaSal | |
|---|---|---|---|
| a The data in parentheses are the difference between the values of βC and βLD. | |||
| βLD | 0.95 | 0.35 | 0.50 |
| βC | 1.3 (0.35) | 0.47 (0.12) | 0.50 (0) |
Formation of rod-like micelles
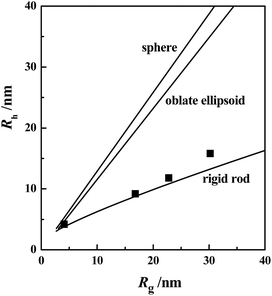
Dynamic, and static, light-scattering were used to determine the hydrodynamic radius, Rh, and the radius of gyration Rg, respectively, for which the measurements were performed at a 90° angle.25,26,29 Fig. 2 plots theoretical Rh versus Rg curves for geometrical models of spheres, oblate ellipsoids, and rigid rods according to the formulae reported elsewhere.30 The experimental data for the C18TABr micelles at nSNphs/nC18TABr = 0.5, 0.6, and 0.7 are shown in Fig. 2, which were close to the theoretical rod line. This indicated that at nSNphs/nC18TABr > 0.47, the micelles had a rod-like shape.The influence of surfactant concentration was also examined at a fixed ratio of nSNphs/nC18TABr (0.5). The results in Fig. 3 show that the experimental points were close to the theoretical rod line except at 100 mmol L−1 at which, deviation from the line was slightly greater. This indicated that the micelles formed by C18TABr over a moderate range of surfactant concentrations were all rod-like in shape.
Why was SNphs chosen, rather than SBzs or NaSal?
Besides SNphs, sodium benzenesulphonate (SBzs) and sodium salicylate (NaSal) were also candidates; the former was the homologue of SNphs, while the latter has been extensively used in C16TABr solution to promote micellar growth.31 Thus, we also tried both salts in the C18TABr system. However, we found that, for the purpose of maintaining the production of rod-like micelles, both SBzs and NaSal may be unsuitable.For SBzs, as shown in Table 1, its efficiencies, including both inhibition of secondary aggregation and promotion of micellar growth, were all far lower than that of SNphs.
For NaSal, although the values of both βLD and βC were comparable with that of SNphs, this salt caused rapid growth of the C18TABr micelles, probably being too strong in its interaction with the surfactant. Thereby, short rod production was hard to maintained, on the contrary, long worm-like micelles were often obtained due to the promotion of NaSal. This conclusion was derived from the comparison of the three salts in the DLS measurements, the micellar shapes (Rh versus Rg plots) and the rheological behaviour (steady-state viscosity curves, which were sensitive to micellar shape32), which are available in ESI, Fig S2 to S7.†
Herein, we would like to re-emphasise that SNphs, rather than NaSal, was chosen to induce rod-like micelles in the C18TABr system. Indeed, NaSal was used more extensively than SNphs in promoting the micellar growth of quaternary ammonium surfactants, and particularly C16TABr, a cationic surfactant studied extensively.33–35 NaSal has been demonstrated to be quite effective at inducing the formation of worm-like micelles but it may be unsuitable if only short rods were required. Contrarily, SNphs can interact, to some extent, with C18TABr, which can ensure the formation of rod-like micelles in the solution.
Solubilisation of styrene
The solubilisation of styrene in the rod-like micelles of C18TABr was examined, herein the templating system was that at nSNphs/nC18TABr = 0.5. Fig. 4 shows the appearance of the samples of C18TABr (30 mmol L−1) in ethanol/water (10/90 wt%) in the presence of SNphs (15 mmol L−1, i.e., nSNphs/nC18TABr = 0.5) and with different mole ratios of styrene to surfactant (nst/nsurf). With increasing nst/nsurf, the sample changed from being clear in appearance (shown as the first region with nst/nsurf < 0.8) to a weak milk-white colour (the second region, 0.8 ≤ nst/nsurf < 1.7), and then completely white, and even slight cloudy (the third region, 1.7 < nst/nsurf < 4.5) (Fig. 4). For the first region where four transparent samples are shown, the hydrodynamic radius, Rh, measured at a 90° angle was represented: it had an average value of 9.2 nm.To confirm the effects of solubilisation, the UV spectra of styrene in the solutions were measured (Fig. 5). The spectrum of styrene (without surfactant) in ethanol/water (10/90 wt%) showed bimodal bands of absorbance over the UV region at 202 nm and 246 nm, respectively: this was similar to that of styrene in methanol–water solvent, which had bimodal bands at 210 nm and 240 nm.36 These have been assigned to the π → π* transition in the vinyl group and the π → π* transition in the aromatic ring. The spectrum of styrene in n-dodecane was a single broad band at around 244 nm (Fig. 5), agreeing with that found in the spectrum of n-decane.36 For styrene in the surfactant solutions, the spectra only showed a single band at 244 nm, which demonstrated that the styrene was solubilised in the micellar cores composed of hydrophobic hydrocarbon tails.
Styrene polymerisation and micellar freezing
The solubilised styrene monomers were polymerised in situ as described in Experimental Section. Fig. 6 shows the appearance of the samples after polymerisation, in which the samples in the secondary region became completely transparent in comparison with those before polymerisation as shown in Fig. 4, and even those in the third region were also clear. This phenomenon was also observed in other cases, for example, the system of spherical micelles formed by mixed C22TABr/C18TABr (unpublished results). For these clear samples in the secondary region, the value of Rh can be now measured (see Fig. 6). The average value of Rh was almost identical to that in the first region and also to those before polymerisation (Fig. 4), which indicated that the polymerisation of styrene did not change the size of the micelles.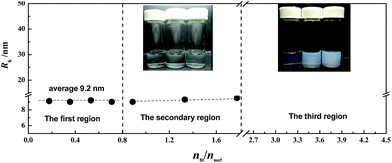 | ||
| Fig. 6 Appearance of the samples in Fig. 4 after polymerisation and the Rh, measured at a 90° angle and processed by the CONTIN model, for those clear samples as a function of nst/nsurf. | ||
SAXS is also often used to analyze the morphology of aggregate. Fig. 7 shows the SAXS scattering intensity (I) as a function of scattering vector (q) (q = (4π/λ)sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where λ is the X-ray wavelength and 2θ is the scattering angle) for the samples before and after polymerisation. The slopes of the double logarithmic plots in the low-q region all had a value of −1 as shown by the solid lines, which indicated the formation of cylindrical (i.e. rod-like) micelles and also demonstrated the micellar morphology unchanged after polymerization.
θ, where λ is the X-ray wavelength and 2θ is the scattering angle) for the samples before and after polymerisation. The slopes of the double logarithmic plots in the low-q region all had a value of −1 as shown by the solid lines, which indicated the formation of cylindrical (i.e. rod-like) micelles and also demonstrated the micellar morphology unchanged after polymerization.
 | ||
| Fig. 7 SAXS spectra (plots of scattering intensity I(q) versus scattering vector q) for the samples at nst/nsurf = 1.3 before (open) and after (solid) polymerization. | ||
The effect of freezing was tested by a dilution experiment, where the samples after polymerisation were diluted 100-fold, i.e., adding 100 g water to 1 g of the sample. After dilution, the samples at low nst/nsurf (the first and secondary regions) showed rather wide distributions of scattering intensity as seen in the insets, which yielded relatively greater average values (represented by the star symbols in Fig. 8, top) compared with the original ones (solid circles). This indicated that the polymerisation did not fix these micelles for these samples and they must be fragmented during dilution. At high nst/nsurf (the third region), however, single, narrow distributions of scattering intensity were obtained for each sample, and in particular, for the last sample (nst/nsurf = 4.4, see inset). Upon increasing the dilution to 400% (a total of 400 g water added to 1 g of the original sample), these samples still showed a single, narrow distribution of intensity (Fig. S11, ESI†). This indicated that at a large nst/nsurf (the third region), the polymerisation can freeze the micelles.
The relative variance, μ2/![[capital Gamma, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ba.gif) 2, obtained by the Cumulant analysis can be used to characterise the polydispersity of aggregates.25,26 The results in Fig. 8 (bottom) showed an average value of 0.08 for μ2/
2, obtained by the Cumulant analysis can be used to characterise the polydispersity of aggregates.25,26 The results in Fig. 8 (bottom) showed an average value of 0.08 for μ2/![[capital Gamma, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ba.gif) 2 at high nst/nsurf (the third region), which indicated a good mono-dispersity for the formed aggregates before, and after, dilution.
2 at high nst/nsurf (the third region), which indicated a good mono-dispersity for the formed aggregates before, and after, dilution.
The aforementioned results suggested an effective approach to freezing the micelles, i.e., a slightly great amount of styrene should be solubilised for this purpose, for example, the sample at nst/nsurf = 4.4 showed good freezing effect. In our unpublished work, the spherical micelles formed by mixed C22TABr/C18TABr were well fixed at nst/nsurf ≥ 4.9. This value was comparable with the present 4.4 (nst/nsurf), which may have entailed a similar mechanism for freezing the aggregates. We also hoped to get some supports from published literature but were disappointed since few reports were available. Here only two cases are shown because they mentioned the values of nst/nsurf related to micellar freezing. Becerra et al. reported a typical value of the weight ratio of styrene (st) to cetyltrimethylammoniumtosilate (CTAT), wst/wCTAT of 0.1, which corresponded to a mole ratio of 0.44, for the CTAT system.7 They suggested that the microstructures of aggregates might be preserved but did not show any evidence to that effect, for example, a dilution test. Kurja et al. reported a rather high value for the weight ratio of styrene to surfactant (dioctadecyldimethylammonium bromide, DODAB) of 1.59, which corresponded to a mole ratio of 9.6, in a DODAB-vesicle system.4 They confirmed that the vesicle structure was retained after the solubilised styrene was polymerised. Comparatively, our two values of nst/nsurf, 4.4 for the present system, and 4.9 for mixed C22TABr/C18TABr spherical micelles, may be more useful because their frozen aggregates have been demonstrated thus by dilution tests.
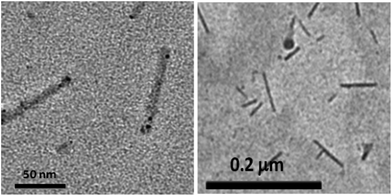
The TEM image of this sample after 100% dilution provided direct observation of the aggregate morphology (Fig. 9), where rod-like micelles were seen. Synthesising all such results, we can now say that, in this system of C18TABr/SNphs (nSNphs/nC18TABr = 0.5), well frozen rod-like micelles have been obtained depending on the polymerisation of solubilised styrene monomers.
Experimental
Materials
C18TABr was synthesised in our laboratory and the purity was checked by 1H NMR and elemental analyses.24 Ethanol (AR, Sinopharm) was used as received. The water used was of Milli-Q grade with a resistivity of 18.2 MΩ cm. SNphs (Acros), sodium benzenesulphonate (SBzs, Aldrich), and sodium salicylate (NaSal, Sinopharm Chemical Reagent Co.) were used as received.Sample preparation
The surfactant was dissolved in ethanol and the solution was filtered through a 0.22 μm pore-size filter. The Milli-Q water was slowly added to the solution using a drop-rate auto-controllable micro-syringe (LongerPump TJ-1A) at a flow rate of 20 μL min−1 until a 90 wt% water content was reached. This method of preparation ensured both homogeneous solutions of C18TABr, and good dissolution of hydrotropes, particularly SNph. The solutions were stirred for 24 h before testing. All experiments were performed at 30 °C.Styrene solubilisation and polymerisation
Reagent grade styrene (AR, Sinopharm) was washed with 10 wt% NaOH several times and then washed with water until the solution pH reached 7 to remove the inhibitor and any polymeric residue. Desired amounts of styrene were put into test tubes containing surfactant micelles in ethanol/water solvent in the presence of organic salt. The test tubes were vibrated for 48 h at a constant 30 ± 0.1 °C to ensure the solublilising equilibrium of styrene in the micelles.The UV absorption spectra of the styrene-containing solutions were recorded on a Hitachi U-3010 (Japan) UV/vis spectrophotometer using a quartz cell with a 5 mm path length.
Polymerisation was carried out in a 250 mL glass reactor with a solution of C18TABr micelles solubilised by styrene. The glass reactor was equipped with a temperature controller and was stirred using a magnetic stirrer. K2S2O8 of 1 wt% with respect to the monomer was added and the solution was heated to 70 °C while being stirred. The reaction was performed for 8 h, after which the temperature was rapidly decreased to room temperature.
Light-scattering measurements
The light-scattering ability of the solutions was measured with a Brookhaven Instrument which was composed of a BI-200SM goniometer, a BI-9000AT digital correlator (522 channels), and a photomultiplier detector. A green laser with 532 nm wavelength and 200 mW output power was used as the light source. The measurement temperature was controlled by a thermostatic circulator (Poly-Science, USA) to an accuracy of ±0.01 °C.For dynamic light-scattering (DLS), the experimental data were analysed by use of the CONTIN program to obtain the intensity-fraction distributions around each characteristic aggregate size. For those double distributions of scattering intensity, a double-exponential model (Dblexp) was also used to analyse the data to a greater precision.25,26
For static light-scattering (SLS), the reduced scattering intensity KC/Rθ was measured at different concentrations. Here, Rθ is the Rayleigh ratio obtained by calibration measurements with benzene: Rθ = 8.51 × 10−6 at 25 °C,27 C is the surfactant concentration, and K is the optical constant which is given by:
| K = 4π2n20(dn/dC)2/(λ40NA) | (1) |
SAXS measurements
The experiments were performed at 25 °C using a NanoStar (Bruker) SAXS equipped with a 2-d detector. The incident X-rays of CuKα radiation (1.54 Å) were monochromated by a cross-coupled Göbel mirror and passed through the sample placed in a 2 mm quartz capillary. The distance between the sample and the detector was 1070 mm, allowing the value of the scattering vector q to range from 0.07 to 2.3 nm−1. The data shown were for the normalised intensity I (arbitrary units) versus q = (4π/λ)sin(θ), where λ is the wavelength of the X-rays and 2θ is the scattering angle.Cryo-EM measurement
A drop (3.5 μL) of solution was applied onto a 300-mesh GiG carbon grid (Jiangsu Life-Trust) that was pre-treated in plasma cleaner (PDC-32G, Harrick Plasma). The grid was then blotted for 3.0 s at 100% humidity, using an FEI Vitrobot (Mark IV) before it was rapidly frozen in liquid ethane that had been cooled by liquid nitrogen. The samples were then imaged with a FEI Titan Krios cryo-EM that was operated at 300 kV and equipped with a Gatan UltraScan 4000 charge-coupled device camera.Conclusions
In this study, we prepared rod-like micelles from C18TABr with the help of SNphs, and further froze their microstructure via solubilising styrene monomers into the micelles and then in situ polymerisation thereof. The main conclusions may be drawn as follows:(1) Depending on assistance from the SNphs, C18TABr formed rod-like micelles. If only a rod-like morphology was required, SNphs were the most suitable for this purpose, and they cannot be substituted by NaSal.
(2) The microstructure of the rod-like micelles formed by C18TABr at nst/nsurf > 2.7 can be well frozen via the polymerisation of solubilising styrene monomers.
(3) To achieve a good freezing effect, a relatively large amount of solubilised styrene may be required: in the present case, for instance, the sample at nst/nsurf = 4.4 exhibited good micellar freezing.
Acknowledgements
Support from The National Natural Science Foundation of China (Grant no. 21273040) is gratefully acknowledged.Notes and references
- D. F. Evans and H. Wennerstrom, The Colloidal Domain, Wiley-VCH, New York, 2001 Search PubMed.
- C. Tanford, The Hydrophobic Effects: Formation of Micelles and Biological Membranes, Wiley, New York, 2nd edn, 1980 Search PubMed.
- J. Israelachvili, Intermolecular & Surface Forces, Academic Press, San Diego, 1992 Search PubMed.
- J. Kurja, R. J. M. Nolte, I. A. Maxwell and A. L. German, Polymer, 1993, 34, 2045–2049 CrossRef CAS.
- J. D. Morgan, C. A. Johnson and E. W. Kaler, Langmuir, 1997, 13, 6447–6451 CrossRef CAS.
- C. A. McKelvey, E. W. Kaler, J. A. Zasadzinski, B. Coldren and H.-T. Jung, Langmuir, 2000, 16, 8285–8290 CrossRef CAS.
- F. Becerra, J. F. A. Soltero, J. E. Puig, P. C. Schulz, J. Esquena and C. Solans, Colloid Polym. Sci., 2003, 282, 103–109 CAS.
- Y. Okazaki, H. Jintoku, M. Takafuji, R. Oda and H. Ihara, RSC Adv., 2014, 4, 33194–33197 RSC.
- A. Guyot, Curr. Opin. Colloid Interface Sci., 1996, 1, 580–586 CrossRef CAS.
- W. Srisiri, T. M. Sisson, D. F. O'Brien, K. M. McGrath, Y. Han and S. M. Gruner, J. Am. Chem. Soc., 1997, 119, 4866–4873 CrossRef CAS.
- S. R. Kline, Langmuir, 1999, 15, 2726–2732 CrossRef CAS.
- S. J. Candau, E. Hirsch, R. Zana and M. Adam, J. Colloid Interface Sci., 1988, 122, 430–440 CrossRef CAS.
- S. J. Candau, E. Hirsch, R. Zana and M. Delsanti, Langmuir, 1989, 5, 1225–1229 CrossRef CAS.
- F. Quirion and L. J. Magid, J. Phys. Chem., 1986, 90, 5435–5441 CrossRef CAS.
- K. R. Francisco, M. A. da Silva, E. Sabadini, G. Karlsson and C. A. Dreiss, J. Colloid Interface Sci., 2010, 345, 351–359 CrossRef CAS PubMed.
- T. Shikata and H. Hirata, Langmuir, 1987, 3, 1081–1086 CrossRef CAS.
- T. Shikata, H. Hirata and T. Kotaka, Langmuir, 1988, 4, 354–359 CrossRef CAS.
- R. Makhloufi, E. Hirsch, S. J. Candau, W. Binana-Limbele and R. J. Zana, Phys. Chem., 1989, 93, 8095–8101 CrossRef CAS.
- A. A. Ali and R. Makhloufi, Phys. Rev. E, 1997, 56, 4474–4478 CrossRef.
- A. A. Ali and R. Makhloufi, Colloid Polym. Sci., 1999, 277, 270–275 CAS.
- T. M. Clausen, P. K. Vinson, J. R. Minter, H. T. Davis, Y. Talmon and W. G. J. Miller, Phys. Chem., 1992, 96, 474–484 CrossRef CAS.
- V. K. Aswal, P. S. Goyal and P. Thiyagarajan, J. Phys. Chem. B, 1998, 102, 2469–2473 CrossRef CAS.
- J. X. Zhao, X. Z. Xu, W. J. Dong and H. B. Yu, Colloids Surf., A, 2015, 484, 253–261 CrossRef CAS.
- J. X. Zhao, W. J. Dong and X. Z. Xu, J. Solution Chem., 2016, 45, 126–139 CrossRef CAS.
- W. Schärtl, Light scattering from polymer solutions and nanoparticle dispersions, Springer, 2007 Search PubMed.
- B. Chu, Light Scattering, Basic principles and practice, Academic Press, Inc, New York, 2nd edn, 1991 Search PubMed.
- E. R. Pike, W. R. M. Pomeroy and J. M. J. Vaughan, Chem. Phys., 1975, 62, 3188–3192 CAS.
- J. X. Zhao, X. Z. Xu, W. J. Dong and H. B. Yu, Colloids Surf., A, 2015, 484, 253–261 CrossRef CAS.
- Z.-J. Yu and R. D. Neuman, Langmuir, 1992, 8, 2074–2076 CrossRef CAS.
- W. V. D. Sande and A. J. Persoons, Phys. Chem., 1985, 89, 404–406 CrossRef.
- C. A. Dreiss, Soft Matter, 2007, 3, 956–970 RSC.
- X. M. Pei, J. X. Zhao, Y. Z. Ye, Y. You and X. L. Wei, Soft Matter, 2011, 7, 2953–2960 RSC.
- T. Shikata, H. Hirata and T. Kotaka, Langmuir, 1987, 3, 1081–1086 CrossRef CAS.
- T. Shikata, H. Hirata and T. Kotaka, Langmuir, 1989, 5, 398–405 CrossRef CAS.
- X. M. Pei, J. X. Zhao and X. L. Wei, Colloids Surf., A, 2010, 366, 203–207 CrossRef CAS.
- P. C. Schulz, R. M. Minardi and B. Vuano, Colloid Polym. Sci., 1998, 276, 278–281 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18794c |
| This journal is © The Royal Society of Chemistry 2016 |