Stable solar-driven water splitting by anodic ZnO nanotubular semiconducting photoanodes†
Abstract
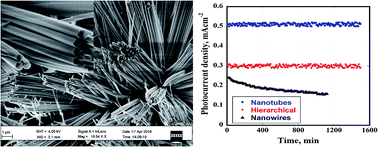
The development of high performance artificial photosynthetic devices, to store solar energy in chemical bonds, requires the existence of stable light-absorbing electrodes for both the oxidative and reductive half-reactions. The development of such systems has been hindered in part by the lack of semiconducting photoanodes that are stable under the water spitting conditions. We demonstrate, for the first time, the synthesis of ZnO nanotubes via anodization of Zn foil and their use as photoanodes in photoelectrochemical hydrogen generation systems. Structural, optical and photoelectrochemical measurements showed the superiority of the nanotubular structure over the nanowire and hierarchical counterparts.

 Please wait while we load your content...
Please wait while we load your content...