Shape-selective synthesis of Bi2WO6 hierarchical structures and their morphology-dependent photocatalytic activities†
Hua Lvab,
Yumin Liu*ab,
Jing Guangab,
Zhiwei Dingab and
Jianji Wang*ab
aCollaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, Henan Normal University, Xinxiang, Henan 453007, P. R. China. E-mail: hualv2009@163.com; jwang@htu.cn; Fax: +86 373 3326336; Tel: +86 373 3326335
bHenan Key Laboratory of Green Chemical Media and Reactions, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, P. R. China
First published on 17th August 2016
Abstract
Various morphologies of Bi2WO6 including packed nanoplates, three dimensional (3D) hierarchical flower-like and clew-like microspheres were successfully synthesized through a simple hydrothermal route using L-lysine as a structure-directing agent. It was found that the morphological modulation of the as-prepared products could be easily realized by adjusting the solution pH values of the precursors. On the basis of a series of time-dependent experiments, the possible mechanisms for the formation of three different structures were proposed. The photocatalytic activities of the different morphologies of Bi2WO6 were evaluated by photodegradation of Rhodamine B (RhB) solution under visible light irradiation (λ > 400 nm). In comparison with clew-like and plate-like Bi2WO6 structures, Bi2WO6 with a hierarchical flower-like architecture exhibited the highest visible-light-photocatalytic activity. The enhanced photocatalytic activity could be mainly ascribed to the unique structural features of flower-like microspheres with hierarchical architectures.
1. Introduction
Over the past few decades, low-dimensional nanostructured materials, such as nanoparticles, nanotubes, nanoribbons, nanowires and nanosheets, have attracted great interest owing to their novel optical/electrical properties and promising applications in the fields of the environment and energy.1–3 In particular, large-scale self-assembly of low-dimensional nano-sized building blocks into 3D complex hierarchical structures represents a hot topic in material chemistry.4,5 The hierarchical structures of highly ordered micro/nanostructures not only possess the excellent physical and chemical properties of the single nano-sized building block but also hold newer and/or better properties resulting from the different arrangements of building blocks. To date, a wide variety of functional materials with hierarchical architectures, including metals, nonmetals, copolymers, organic–inorganic hybrid materials, biomaterials and semiconductors, have been successfully synthesized on the basis of different driving mechanisms.6–8Bismuth tungstate (Bi2WO6), as one of the simplest members of the Aurivillius oxides, has attracted extensive attention owing to its excellent photocatalytic performance under visible light irradiation.9–12 As is well known, the photocatalytic activity is closely interrelated with the size, morphology and structure of photocatalyst. Stimulated by morphology- or size dependent properties, Bi2WO6 with diverse structures such as nanoparticles, nanosheets, nanooctahedra, microdiscs, nanocages, flower-like, tyre and helix-like, tubes and spheres, have been successfully prepared through a variety of methods.9,13–19 Among the various nanostructures, Bi2WO6 with 3D hierarchical microsphere structures constructed by nanoplates have been demonstrated to possess superior photocatalytic activity, as the pores with different sizes among the nanoplates are beneficial for reactants transport and photo-energy harvesting.20,21 Generally, Bi2WO6 with spherical architectures are prepared by template-assisted routes using soft (e.g., organic surfactants or emulsified micelles) or hard templates (e.g., polymeric colloidal particles, SiO2 or carbon spheres) to control the processes of nucleation, growth and alignment.9,22 In addition to template-assisted process, Huang et al.21 and Amano et al.23 synthesized hierarchical Bi2WO6 microspheres by a facile hydrothermal route without using any surfactant or template as structure-directing agent. Mann et al.24 and Huang et al.25 also fabricated uniform porous Bi2WO6 microspheres via the ultrasonic spray pyrolysis method. However, despite these remarkable progresses, it is still a great challenge to explore simple and facile approaches to synthesize homogeneous Bi2WO6 microspheres with hierarchical architectures.
In this contribution, we describe a facile and universal hydrothermal method to synthesize various morphologies of micro/nanostructured Bi2WO6 by employing environment-friendly L-lysine, a nontoxic biomolecules, as structure-directing agent. The effects of pH values and the assistance of L-lysine on the morphological variation were investigated in detail. On the basis of the time-dependent experiments, the possible formation mechanisms of different Bi2WO6 structures were discussed. The photocatalytic activities of the obtained Bi2WO6 with different structures were evaluated and the results indicated that the hierarchical flower-like Bi2WO6 microspheres exhibited superior visible-light-driven photocatalytic activity over the other Bi2WO6 structures. Moreover, the relationship between the structural characteristics and their photocatalytic performance was also discussed.
2. Experimental
2.1 Materials and synthesis
All reagents were analytical grade and used without further purification. In a typical procedure, 2.5 mmol of Na2WO4·2H2O was dissolved in 30 mL distilled water, then 0.8 g of L-lysine was added and the obtained mixture was kept stirred for about 1 h to get a homogeneous solution (marked as solution A). Subsequently, 5 mmol of Bi(NO3)3·5H2O was dissolved in 20 mL distilled water containing 2 mL concentrated nitric acid to obtain a transparent solution (marked as solution B). Then, solution B was added into solution A under continuous stirring. The pH value of the precursor suspension was adjusted by 4 mol L−1 nitric acid or 25 wt% aqueous solution of ammonia. After being stirred for another 1 h, the resulting suspension was transferred into a 100 mL Teflon-lined stainless steel autoclave and maintained at 160 °C for 20 h. After that, the autoclave was cooled down to room temperature, and the resulting products were collected by centrifugation, washed several times with distilled water and absolute ethanol, and finally dried at 80 °C for 12 h under vacuum. The synthesized Bi2WO6 samples were denoted as BWO-x (where x is the pH value of the precursor suspension; x – 1, 6 and 11).2.2 Characterization
The crystal structure of the as-synthesized samples was characterized by powder X-ray diffraction (XRD) on a Bruker D8-advance diffractometer with Cu Kα radiation (λ = 0.15406 nm). The morphology of the samples was examined by scanning electron microscopy (SEM, JSM-6390-LV, JEOL). Transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) images were performed on a JEM2100 microscope. A spectrophotometer (Cary5000 UV-vis-NIR) was used to obtain the UV-vis diffuse reflectance spectra of the products in the region of 260 to 600 nm. The photoluminescence (PL) spectra were recorded on a fluorescence spectrophotometer (Shimadzu RF-5301PC) with an excitation wavelength of 300 nm. Nitrogen adsorption/desorption measurements were conducted at 77 K using a Micromeritics ASAP 2010 instrument and the specific surface area was calculated based on Brunauer–Emmett–Teller (BET) method.2.3 Photocatalytic test
Photocatalytic activity of the obtained samples was evaluated by photodegradation of RhB under the irradiation of a 300 W Xe lamp with a UV cutoff filter (λ > 400 nm). All photodegradation processes were conducted in a photoreaction apparatus with temperature being kept at room temperature as described elsewhere.26 Typically, 0.2 g of catalyst was added to 200 mL of RhB aqueous solution (10 mg L−1). Before irradiation, the suspension was magnetically stirred for 30 min in the dark to establish adsorption–desorption equilibrium between the photocatalyst and RhB molecules. Then the suspension was irradiated by the Xe lamp under magnetic stirring. At given time intervals, about 5 mL of suspension was sampled and centrifuged to remove catalyst. The concentration of RhB before and after degradation was monitored with a T6 UV spectrophotometer at λmax of 552 nm.3. Results and discussion
3.1 Structure and morphology
The phase structure and purity of the obtained samples were characterized by XRD technique. Fig. 1 exhibits the XRD patterns of the three Bi2WO6 samples synthesized at pH 1, 6 and 11, respectively. The diffraction peaks of the three samples were indexed well to the pure orthorhombic phase of Bi2WO6 (JPCPDS card no. 73-1126), with lattice constants a = 5.456, b = 5.436, and c = 16.426 Å. No peaks of impurities could be detected, suggesting the high phase purity of the obtained sample. Meanwhile, the diffraction peaks of Bi2WO6 samples became low and broad with decreasing pH value, indicating that low pH value could prevent the crystallite growth and lead to small crystallite size, thereby improving the surface area of Bi2WO6. According to the Scherrer equation, the average crystallite sizes of BWO-1, BWO-6 and BWO-11 particles were calculated to be 11.4, 17.1 and 36.0 nm, respectively, by using the full width at half maximum of the (113) diffraction peak at 28.3°. In addition, the intensity ratios of the (200) or (020) peak to the strongest peak (113) for BWO-1, BWO-6 and BWO-11 samples are 0.593, 0.529 and 0.387, respectively, which are much higher than the value of the standard XRD pattern (0.247), indicating that the as-prepared products are preferentially oriented along the (001) crystallographic plane. Besides, the effect of L-lysine on the crystal structure of Bi2WO6 was investigated, as illustrated in Fig. S1 (ESI†). The shapes of diffraction peaks are similar and the intensities also gradually decrease with the decrease of pH values, indicating that the addition of L-lysine has no obvious effect on the crystal structure of Bi2WO6.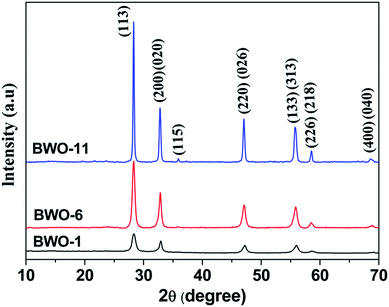 | ||
| Fig. 1 XRD patterns of Bi2WO6 samples synthesized at different pH values in the presence of L-lysine. | ||
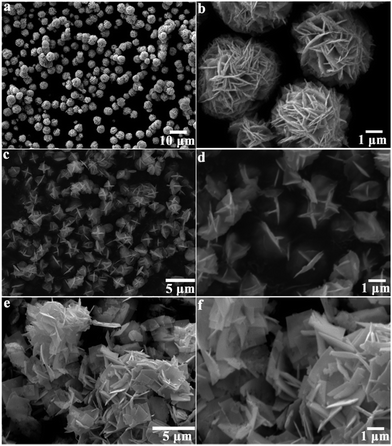
Fig. 2 illustrates SEM images of BWO-1, BWO-6 and BWO-11 samples. It was found that the particle sizes and morphologies of Bi2WO6 samples synthesized at different pH values were quite different. The BWO-1 sample obtained at pH value of 1.0 is composed of a large number of regular and monodisperse microspheres with average diameters of about 5 μm (Fig. 2a). The close-up view of the microspheres indicates that BWO-1 sample possesses the hierarchical flower-like microstructure, which consists of a large quantity of two-dimensional nanoplates with a thickness of about 60 nm. These nanoplates were perpendicularly interwoven together along the radial direction to construct the sphere-shaped structures (Fig. 2b). More importantly, a large number of pores with different diameter sizes could be found among the nanoplates in the microspheres, which might act as transport paths for reactant molecules and consequently improve the physicochemical performance. When the pH value of precursor suspension was increased to 6, the obtained BWO-6 sample also exhibited microsphere-like structure but these microspheres were in a clew-like architecture with average diameter of 2 μm. A close observation revealed that each clew was actually made up of some crisscrossing nanoplates. With further increase of pH value up to 11, the obtained BWO-11 sample exhibited a plate-like structure with average thickness of about 110 nm, while the lateral size reached several micrometers (Fig. 2e and f). Moreover, these nanoplates were closely packed together and the surfaces of the nanoplates were flat and smooth.
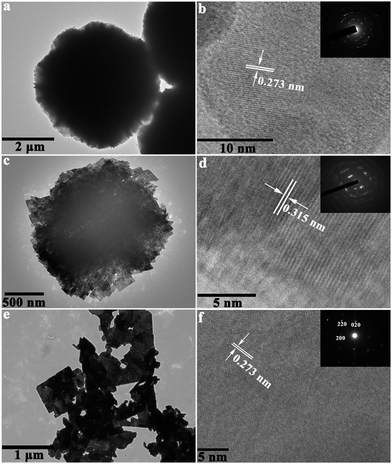
The morphological and structural features of BWO-1, BWO-6 and BWO-11 samples were further investigated by TEM and HRTEM. Fig. 3a displays a representative TEM image of an individual flower-like microsphere with a zigzag brim of nanoplates, which further confirms that 3D hierarchical flower-like architectures are constructed by 2D thin nanoplates, and this is in good consistence with the above SEM results. The lattice interplanar spacing, shown in the HRTEM image (Fig. 3b), was determined as 0.273 nm, corresponding to (200) plane of orthorhombic Bi2WO6. Fig. 3c shows the TEM observation of an individual clew-like microsphere. Obviously, the clew-like microsphere was composed of many interlaced nanoplates. A sharp contrast between different regions could be observed in the TEM image, indicating that the building blocks of nanoplates were loosely packed. As shown in the HRTEM image (Fig. 3d), the spacing of the interplanar lattice was determined as 0.315 nm, corresponding to (113) plane of orthorhombic Bi2WO6. In addition, as shown in the corner of Fig. 3b and d, selective area electron diffraction (SAED) patterns of individual flower-like and clew-like Bi2WO6 microspheres suggest polycrystalline structures. Fig. 3e and f show the TEM and HRTEM images of BWO-11 sample, respectively. The as-prepared BWO-11 sample exhibited plate-like rectangular shape and the adjacent lattice spacing was 0.273 nm, which corresponded to (200) plane of orthorhombic Bi2WO6. The SAED pattern (inserted of Fig. 3f) for the (001) zone axis displayed its single crystallite structure and confirmed that the as-prepared nanoplates preferentially grew along the (001) plane, which was parallel to the instinct a × b layer plane.
Moreover, it should be noted that the addition of L-lysine has a significant effect on the morphology of the as-prepared samples. Fig. S2† demonstrates the morphological variation of the samples without using L-lysine. Obviously, without the assistance of L-lysine, all of the as-prepared three Bi2WO6 samples are composed of irregular plate-like or aggregate structures, indicating that L-lysine used as a structure-directing agent can adjust the crystal growth and plays key role in morphological modulation of Bi2WO6 products.
3.2 Morphological evolution
To understand growth mechanisms of the three typical Bi2WO6 structures, a series of time-dependent experiments were carried out and the products obtained at different growth stages were then investigated by XRD analysis and SEM observation. Fig. S3† shows the XRD patterns of flower-like Bi2WO6 microspheres prepared at different reaction time. As shown in Fig. S3,† the weak peaks were observed in the initial stage (1 h), demonstrating its amorphous characteristics. When the reaction time was extended to 2 h, the diffraction peaks became strong, indicating that crystalline Bi2WO6 was formed at this stage. As the reaction time was further prolonged, the diffraction peaks intensities improved gradually, suggesting the increased crystallinity of the products. Fig. 4 shows the typical SEM images of flower-like Bi2WO6 microspheres prepared at different reaction time. After 1 h of hydrothermal treatment (Fig. 4a), only bulk agglomerates composed of small nanoparticles were obtained. When the reaction time was prolonged to 2 h (Fig. 4b), the products exhibited sphere-like microspheres with average diameter of about 4 μm, coexisting with the nanoparticles. After 6 h of hydrothermal treatment (Fig. 4c), spherical superstructures could be found, but these superstructures were still at their intermediate stage. Further increasing the reaction time to 12 h (Fig. 4d), these superstructures were gradually ripened and the building blocks became more distinct. Finally, well-defined and regular flower-like Bi2WO6 microspheres could be obtained at 20 h (Fig. 2a and b). | ||
| Fig. 4 SEM images of flower-like Bi2WO6 microspheres prepared at different hydrothermal time: (a) 1 h, (b) 2 h (c) 6 h (d) 12 h. | ||
The XRD patterns and SEM images of the time-dependent evolution of the clew-like Bi2WO6 were shown in Fig. S4 and S5,† respectively. As shown in Fig. S5a and b,† the products exhibited bulk agglomerates and sphere-like structures after hydrothermal treatment for 1 h and 2 h, respectively, which was similar to the evolution process of the flower-like Bi2WO6 microspheres. However, as the reaction time was prolonged to 6 h, incomplete clew-like microsphere structures were developed (Fig. S5c†). When the reaction time was extended from 12 to 20 h, most of the obtained products exhibited uniform and well-defined clew-like hierarchical architectures constructed by 2D nanoplates (Fig. S5d† and 2c).
When the solution pH value of the precursor was increased to 11, different phase and morphology evolution process could be observed in the reaction system. As shown in Fig. S6a,† after 1 h of hydrothermal treatment, the obtained products were irregular nanosized particles. Moreover, according to its corresponding XRD patterns (Fig. S7†), the sharp and strong diffractions peaks could be observed at this stage, indicating that well-crystallized Bi2WO6 appeared. When the reaction time was extended from 2 h to 6 h, some laminar structures were formed, coexisting of nanosized particles (Fig. S6b and c†). After 12 h of hydrothermal treatment, the irregular nanoparticles almost disappeared and the obtained nanoplates were further developed (Fig. S6d†). As the reaction time was prolonged to 20 h, good-quality nanoplates with smooth surface could be obtained (Fig. 2e and f).
3.3 Formation mechanism
On the basis of the above experimental results, the possible formation mechanisms of flower-like, clew-like and plate-like Bi2WO6 structures obtained at different pH values were illustrated in Fig. 5. For flower-like Bi2WO6 structure, when Bi(NO3)3·5H2O and Na2WO4·2H2O were mixed together along with L-lysine, irregular nanoparticles were firstly formed. Then the carboxyl and amine groups of L-lysine could coordinate with Bi3+ ions to produce the intermediate complex of Bi3+-L-lysine, thereby significantly decreasing the concentration of free Bi3+ ions.27 Such a low concentration of Bi3+ made the reaction rate slow down and led to the separation of nucleation and growth process, which facilitated the growth of good-quality crystal in consideration of the kinetic process. It is well known that, although the formation of smaller crystallites is favored during the initial agglomeration in view of kinetic process, larger crystallites are thermodynamically favored.17 Thus, to reduce the surface energy of the system, the formed irregular nanoparticles tended to self-aggregate into sphere-like microparticles with prolonged hydrothermal treatment (Fig. 5, step 1). Additionally, many free L-lysine molecules might be absorbed on the surface of sphere-like microparticles, which would supply many high-energy nucleation sites for nanocrystalline growth. With further increase in the hydrothermal treatment, the crystallization of Bi2WO6 nanocrystals begins from these high-energy nucleation sites anchoring with L-lysine molecules. Then sphere-like microparticles gradually grow and subsequently dissolve these nanocrystals from the outer side of the aggregates toward the inside. This dissolution–recrystallization process is a universal phenomenon during the crystal growth process and also known as Ostwald ripening (Fig. 5, step 2). Meanwhile, due to the high intrinsic anisotropic nanoplates were formed. In addition, the free L-lysine molecules were prefer to absorb on these primary nanoplates and acted as characteristics of Bi2WO6, these nanocrystals preferentially grow into plate-like structures rather than nanoparticles. In a further crystallization process, the spherical microparticles were exhausted due to mass diffusion and Ostwald ripening, and primary Bi2WO6 potential crystal face inhibitors in the growth process, which is favorable for the formation of oriented nucleation and the self-assembly of the as-formed nanoplates, thereby resulting in the construction of a hierarchical flower-like architecture built form the well-ordered and oriented nanoplates (Fig. 5, step 3).Interestingly, the growth mechanism of the clew-like Bi2WO6 structure is similar to that of flower-like Bi2WO6 structure and the possible reasons are discussed later. As for plate-like Bi2WO6 structure, tiny crystalline nuclei were formed in the beginning and then the crystal growth was followed. Based on the well-known Gibbs–Thomson law, the bigger particles grow at the cost of the smaller ones because of solubility difference between the bigger and smaller particles.28 The orthorhombic Bi2WO6 was constructed by alternating (Bi2O2)2+ layers and (WO4)2− layers, and the layers were parallel to the (001) facets.10 Due to the high intrinsic anisotropic nature of Bi2WO6, the formed Bi2WO6 crystals were prefer to develop plate-like structures through anisotropic growth parallel to the layer. Thus, the coexistence of small plate-like structure and irregular particles were observed in early stages (Fig. S6b and c†). With further increase of hydrothermal treatment, irregular nanoparticles disappeared and only plate-like structures were existed in the products (Fig. S6d† and 2e), confirming that the nanoplates grew at the cost of the smaller nanoparticles.
In this work, the solution pH value of the precursor along with the assistance of L-lysine plays a vital role in the formation of Bi2WO6 with diverse structures. When the pH value of the precursor was low (pH = 1.0), Bi3+ was the main form of bismuth, and the amine groups of L-lysine with lone pairs acted as the main complexing agent to coordinate with Bi3+ cations, resulting in the subsequent nucleation, crystallization and self-assemble of the formed building blocks.29 When the precursor suspension was adjusted to be weakly acidic (pH = 6), the hydrolysis reaction of Bi(NO3)3 was carried out and the resulting BiO+ cations were the main form of bismuth in the suspension.30 Thus, the chelation reaction between the BiO+ cations and the amine and/or carboxyl groups of L-lysine was occurred. Then, during the dissolution–recrystallization process, some free L-lysine molecules would absorb on the Bi2WO6 surfaces and provide active sites for nanocrystalline growth. At this stage, the number of active sites on Bi2WO6 surfaces might be decreased because of the variation of pH value and/or the formation of bismuth ions, which would affect the dissolution–recrystallization process and make the obtained Bi2WO6 superstructures become looser, thereby leading to the clew-like structures. When the pH value of the precursor suspension was increased to 11, a Bi(OH)3 precipitate would be formed immediately and few bismuth ions could coordinate with L-lysine molecules to form the intermediate complex, which would lead to the rapid nucleation and growth process, and finally form the plate-like structure due to the intrinsic anisotropic crystallographic nature of Bi2WO6. However, the reasons for the construction of Bi2WO6 with diverse morphologies are not yet well-understood and further investigation is needed to elucidate the detailed formation mechanisms of these mentioned structures.
3.4 Porous structure and BET surface area
The surface area and porosity of the as-prepared BWO-1, BWO-6 and BWO-11 products were analyzed by N2 adsorption–desorption isotherms. As shown in Fig. 6, BWO-11 sample can be assigned as type III isotherm according to the IUPAC classification, which characteristically suggests that weak interaction occurs between the sample and N2 molecules. This phenomenon may be related with its regular plate-like structure, as revealed by SEM observation. Nevertheless, the isotherms corresponding to BWO-6 and BWO-1 samples are of type IV with distinct H3 hysteresis loops in the p/p0 range of 0.7–1.0, indicating the presence of slit-shaped pores.31 Moreover, both BWO-6 and BWO-1 samples show high absorption at high p/p0 range and no saturated adsorption can be reached till p/p0 = 1.0, suggesting the formation of large mesopores and macropores.32,33 These observations were further verified by the corresponding pore size distribution curves (inset of Fig. 6), in which a wide pore size distribution from the mesoporous to macroporous region could be found. As revealed by SEM observation, the porous structures of BWO-1 and BWO-6 were mainly formed between the intercrossed Bi2WO6 nanoplates. The BET surface areas of BWO-1, BWO-6 and BWO-11 samples were calculated to be 37.9, 18.7 and 4.9 m2 g−1, respectively. Generally, the porous structure and large surface area are expected to be beneficial for the photocatalytic process by providing efficient transport pathways for reactants and facilitating the absorption of contaminants for photodegradation.34 Therefore, a structure-associated improvement can be expected in the photocatalytic activities of the prepared Bi2WO6 samples. | ||
| Fig. 6 N2 adsorption/desorption isotherms and the corresponding pore size distribution curves (inset) of samples. | ||
3.5 Optical property
The optical property of the as-prepared Bi2WO6 with different morphologies was characterized by UV-vis diffuse reflectance spectroscopy (Fig. 7). According to the spectrum, one can see that all the obtained Bi2WO6 samples displayed absorption from UV to visible light regions and the onset of absorption region was around 440 nm, which is in accordance with the band gap absorption reported in previous study.11 However, careful observation reveals that BWO-1 and BWO-6 samples exhibited intensive optical responses compared to that of BWO-11 sample, which might be ascribed to the nano-sized effects and/or transitions from the impurity level generated from the distortion of crystal face and lattice defect.35 As shown in the inset of Fig. 7, the band gap energies (Eg) of the as-synthesized Bi2WO6 samples could be calculated from the plots of (αhν)2 versus photon energy (hν).21 By extrapolating the straight portions of (αhν)2 − hν plots to X axis, the band gaps of BWO-1, BWO-6 and BWO-11 samples were estimated to be 2.94 eV, 2.96 eV and 3.04 eV, respectively. In photocatalytic process, the smaller band gap usually means more visible light harvest. Thus, it is predicted that the hierarchical flower-like and clew-like Bi2WO6 may possess the relatively higher photocatalytic activity than that of plate-like Bi2WO6 under visible light irradiation.Because PL emission mainly arises from the radiative recombination of the excited electrons and holes, PL spectra can be used to evaluate the recombination chance of the photoinduced charge carriers in a semiconductor. Generally, the high PL emission intensity implies the high recombination chance of the photoinduced electrons and holes.36 The comparison of PL emission spectra of BWO-1, BWO-6 and BWO-11 samples is shown in Fig. 8. It is found that the PL emission spectra of the Bi2WO6 samples with different morphologies show the main peaks at the same positions but with different intensities. The strongest peaks centred at 465 nm is ascribed to the inherent luminescence of Bi2WO6, which derives from the direct charge transfer of band transition between the hybrid orbital of Bi6s and O2p (valance band) to the empty W5d orbital (conduction band).37 The broad peak at 530 nm is ascribed to point defects in Bi2WO6 crystals (i.e., oxygen vacancies and metal atom defects).24 Moreover, one can see that the PL emission intensity of BWO-11 was much lower compared to BWO-1 and BWO-6, indicating that the recombination chance of photoinduced electrons and holes was inhibited in BWO-11 sample. This may be attributed to the high crystallinity and single-crystalline nature (i.e., no grain boundaries) of BWO-11 as revealed by XRD and HRTEM results, which lead to less number of defects, thereby resulting in the lower PL intensity.24 On the other hand, the higher PL intensity also means more quantity of electrons and holes were produced, which was beneficial for the photocatalytic oxidative reactions.4
3.6 Photocatalytic activity
As we know, due to the different morphologies and structures, the properties of materials with the same composition could be significantly different. The photocatalytic activities of the as-prepared Bi2WO6 products with different morphologies were evaluated as photocatalysts for the degradation of RhB solution under visible light irradiation. Fig. 9 shows the variation of RhB concentration with irradiation time for BWO-1, BWO-6 and BWO-11, respectively. For the sake of comparison, a blank test (without any Bi2WO6 photocatalyst) was also carried out under the identical conditions, and the result suggested that the direct photolysis of RhB was negligible under the visible light irradiation. In contrast, the presence of photocatalysts resulted in obvious photodegradation of RhB, and the photodegradation efficiencies of different catalysts depended on their morphologies. Obviously, the flower-like Bi2WO6 (BWO-1) exhibited superior photocatalytic activity over clew-like and plate-like ones. Besides, the photocatalytic activities of Bi2WO6 samples obtained in the absence of L-lysine were also evaluated and the results were illustrated in Fig. S8.† Compared to BWO-1, BWO-6 and BWO-11 catalysts, Bi2WO6 samples obtained in the absence of L-lysine exhibited relatively lower photodegradation efficiencies, which further demonstrated the structure-directing agent induced improvement of the photocatalytic activities.It is well known that the photocatalytic activity is dominated by various factors such as the band gap, surface area, morphology, crystallinity, recombination and excitation of electron–hole pairs. Based on the above results, it can be observed that the flower-like structure Bi2WO6 possesses the smallest band gap and the highest specific surface area. The small band gap means more visible light can be utilized during the photodegradation process, while the high specific surface area results in the high adsorption capability for RhB dye and can provide large contact areas between the RhB molecules and the active sites, thereby enhancing the efficiency of photocatalyst. Besides, the high dye adsorption performance of flower-like Bi2WO6 is beneficial for the dye photosensitization process usually occurred in the degradation of RhB dye molecules, which might also lead to the improvement of the photocatalytic performance.38 Meanwhile, as revealed in Fig. 6, the flower-like Bi2WO6 catalysts have large number of meso- and macro-pores, which will supply efficient transport paths for reactant molecules and thus the photocatalytic reaction can occur more easily and efficiently.39 In addition, the hierarchical flower-like structures can allow multiple scattering of visible light and offer a longer optical path, which results in an enhanced light-harvesting and increases the number of photogenerated charge carriers available for the photocatalytic reactions.40
Besides the above factors, the rate of charge carrier mobility may also significantly affect performance of photocatalysts. For randomly photoinduced charge carriers, the average diffusion time (τ) from the interior particle to the surface is expressed by τ = r2π2D, where r and D is the grain radius and diffusion coefficient, respectively.41 Obviously, the decreasing grain radius is beneficial for improving the rate of the charge carrier mobility. As demonstrated by XRD results, Bi2WO6 with flower-like structure exhibited much smaller crystallite size than that of clew-like and plate-like samples, implying that more quantity of photoinduced electron–hole pairs could effectively migrate to the flower-like Bi2WO6 surface to decompose the absorbed RhB dye molecules. Therefore, the excellent photocatalytic activity of flower-like structure Bi2WO6 could be attributed to the synergetic effects among small band gap, high surface area, porous structure and small crystallite size.
In view of the long-term practical applications of photocatalysts, stability and recyclability are two extremely important aspects. Towards to this end, the cycling runs for the degradation of RhB with flower-like Bi2WO6 catalyst were performed to evaluate its stability and recyclability. As shown in Fig. S9,† no obvious decrease in photocatalytic activity was observed for the flower-like Bi2WO6 after four recycles, which implied that the flower-like Bi2WO6 had good stability and recyclability in the whole photocatalytic process. In addition, XRD patterns and SEM images of the flower-like Bi2WO6 after cycling runs were shown in Fig. S10 and S11,† respectively. Obviously, the crystal structure and morphology of the flower-like Bi2WO6 were not changed, further confirming its excellent stability during the reaction.
4. Conclusions
In summary, a facile and effective L-lysine-assisted hydrothermal route was employed to prepare Bi2WO6 with plate-like, 3D hierarchical clew-like and flower-like structures. It was found that the morphology of the final product varied remarkably with the change of the pH value of the precursor suspension. The formation mechanisms of different Bi2WO6 structures were investigated and the results indicated that the flower-like and clew-like Bi2WO6 superstructures might be formed through self-aggregation, Ostwald ripening and self-assembly processes, while the plate-like Bi2WO6 was formed through the anisotropic growth of the formed nanoparticles. Due to the synergetic effects such as small band gap, high surface area, porous structure and small crystallite size, the flower-like Bi2WO6 microspheres exhibited superior activity performance over clew-like and plate-like Bi2WO6 samples for degradation of RhB dye under visible light irradiation (λ > 400 nm). This work not only provides an efficient approach for the selectively controllable synthesis of Bi2WO6 material, but also gives some insight into the design of multicomponent oxide semiconductors with controllable morphology and improved photocatalytic activities.Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (Grant No. U1204503) and Key projects of science and technology of Henan Educational Committee (Grant No. 14B150049, 2010A150014).Notes and references
- M. Cargnello, T. R. Gordon and C. B. Murray, Chem. Rev., 2014, 114, 9319–9345 CrossRef CAS PubMed.
- B. Zhu, B. Lin, Y. Zhou, P. Sun, Q. Yao, Y. Chen and B. Gao, J. Mater. Chem. A, 2014, 2, 3819–3827 CAS.
- W. Li, C. Li, B. Chen, X. Jiao and D. Chen, RSC Adv., 2015, 5, 34281–34291 RSC.
- H. Sun, Y. Yu, J. Luo, M. Ahmad and J. Zhu, CrystEngComm, 2012, 14, 8626–8632 RSC.
- S. Wang, Z. Zheng, B. Huang, Z. Wang, Y. Liu, X. Qin, X. Zhang and Y. Dai, RSC Adv., 2013, 3, 5156–5161 RSC.
- J. Zhao, H. Lai, Z. Lyu, Y. Jiang, K. Xie, X. Wang, Q. Wu, L. Yang, Z. Jin, Y. Ma, J. Liu and Z. Hu, Adv. Mater., 2015, 27, 3541–3545 CrossRef CAS PubMed.
- T. Han, Y. Chen, G. Tian, J.-Q. Wang, Z. Ren, W. Zhou and H. Fu, Nanoscale, 2015, 7, 15924–15934 RSC.
- R. G. Weiner and S. E. Skrabalak, Angew. Chem., Int. Ed., 2015, 54, 1181–1184 CrossRef CAS PubMed.
- T. Sivakumar Natarajan, H. C. Bajaj and R. J. Tayade, CrystEngComm, 2015, 17, 1037–1049 RSC.
- L. Xu, X. Yang, Z. Zhai and W. Hou, CrystEngComm, 2011, 13, 7267–7275 RSC.
- D. He, L. Wang, H. Li, T. Yan, D. Wang and T. Xie, CrystEngComm, 2011, 13, 4053–4059 RSC.
- L. Liu, Y. Qi, J. Lu, S. Lin, W. An, J. Hu, Y. Liang and W. Cui, RSC Adv., 2015, 5, 99339–99346 RSC.
- V. D. Nithya, R. K. Selvan, L. Vasylechko and C. Sanjeeviraja, RSC Adv., 2014, 4, 4343–4352 RSC.
- Q. Sun, X. Jia, X. Wang, H. Yu and J. Yu, Dalton Trans., 2015, 44, 14532–14539 RSC.
- Z. Lou, J. N. Deng, L. L. Wang, L. J. Wang and T. Zhang, Sens. Actuators, B, 2013, 182, 217–222 CrossRef CAS.
- M. Shang, W. Wang and H. Xu, Cryst. Growth Des., 2009, 9, 991–996 CAS.
- L. Zhang, W. Wang, Z. Chen, L. Zhou, H. Xu and W. Zhu, J. Mater. Chem., 2007, 17, 2526–2532 RSC.
- L. Zhang, W. Wang, L. Zhou and H. Xu, Small, 2007, 3, 1618–1625 CrossRef CAS PubMed.
- S.-J. Liu, Y.-F. Hou, S.-L. Zheng, Y. Zhang and Y. Wang, CrystEngComm, 2013, 15, 4124–4130 RSC.
- F. Amano, K. Nogami and B. Ohtani, J. Phys. Chem. C, 2009, 113, 1536–1542 CAS.
- H. Huang, H. Chen, Y. Xia, X. Tao, Y. Gan, X. Weng and W. Zhang, J. Colloid Interface Sci., 2012, 370, 132–138 CrossRef CAS PubMed.
- M. Ge, Y. Li, L. Liu, Z. Zhou and W. Chen, J. Phys. Chem. C, 2011, 115, 5220–5225 CAS.
- F. Amano, K. Nogami, R. Abe and B. Ohtani, J. Phys. Chem. C, 2008, 112, 9320–9326 CAS.
- A. K. P. Mann, E. M. P. Steinmiller and S. E. Skrabalak, Dalton Trans., 2012, 41, 7939–7945 RSC.
- Y. Huang, Z. Ai, W. Ho, M. Chen and S. Lee, J. Phys. Chem. C, 2010, 114, 6342–6349 CAS.
- Y. Liu, L. Hua and S. Li, Desalination, 2010, 258, 48–53 CrossRef CAS.
- H.-J. Kim, K.-I. Choi, A. Pan, I.-D. Kim, H.-R. Kim, K.-M. Kim, C. W. Na, G. Cao and J.-H. Lee, J. Mater. Chem., 2011, 21, 6549–6555 RSC.
- C. Zhang and Y. Zhu, Chem. Mater., 2005, 17, 3537–3545 CrossRef CAS.
- G. P. Nagabhushana, G. Nagaraju and G. T. Chandrappa, J. Mater. Chem. A, 2013, 1, 388–394 CAS.
- X. Xing, Y. Ma, J. Li, G. Fan, H. Ding, X. Ma, L. Yang and G. Xi, CrystEngComm, 2014, 16, 10218–10226 RSC.
- P. Madhusudan, J. Ran, J. Zhang, J. Yu and G. Liu, Appl. Catal., B, 2011, 110, 286–295 CrossRef CAS.
- J. Yu and J. Zhang, Dalton Trans., 2010, 39, 5860–5867 RSC.
- J. Yu, J. Fan and K. Lv, Nanoscale, 2010, 2, 2144–2149 RSC.
- J. Yu, J. Zhang and S. Liu, J. Phys. Chem. C, 2010, 114, 13642–13649 CAS.
- T. Zhao, J. Zai, M. Xu, Q. Zou, Y. Su, K. Wang and X. Qian, CrystEngComm, 2011, 13, 4010–4017 RSC.
- D. Wang, G. Xue, Y. Zhen, F. Fu and D. Li, J. Mater. Chem., 2012, 22, 4751–4758 RSC.
- Z. Zhang, W. Wang, L. Wang and S. Sun, ACS Appl. Mater. Interfaces, 2012, 4, 593–597 CAS.
- J. Xiong, G. Cheng, G. Li, F. Qin and R. Chen, RSC Adv., 2011, 1, 1542 RSC.
- J. Yu, Y. Su and B. Cheng, Adv. Funct. Mater., 2007, 17, 1984–1990 CrossRef CAS.
- H. Li, Z. Bian, J. Zhu, D. Zhang, G. Li, Y. Huo, H. Li and Y. Lu, J. Am. Chem. Soc., 2007, 129, 8406–8407 CrossRef CAS PubMed.
- M. Shang, W. Wang, S. Sun, L. Zhou and L. Zhang, J. Phys. Chem. C, 2008, 112, 10407–10411 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra14493d |
| This journal is © The Royal Society of Chemistry 2016 |