Facile synthesis of D–π–A structured dyes and their applications towards the cost effective fabrication of solar cells as well as sensing of hazardous Hg(ii)†
Abstract
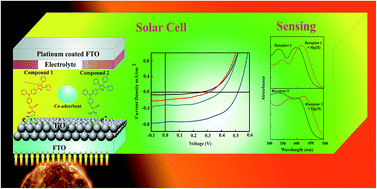
We design and establish an environmentally benign synthesis of new D–π–A dyes containing imidazole derivatives structured to triphenylamine (TPA) as a donor and cyano acrylic groups acting as acceptors without using any toxic catalysts. These newly synthesized imidazole derivatives are anchored on TiO2, towards a cost effective dye sensitized organic solar cell, and achieve 0.22% solar-light to electricity conversion efficiency under standard AM 1.5G irradiation (100 mW cm−2). The most widely significant findings of this study are high thermal stability, smooth surface, strong intramolecular interaction between dyes and TiO2 which improve the performance of the dye sensitized solar cell. The metal ion sensing properties of both the dyes were investigated optically using UV-Vis absorption spectroscopy. The results exhibited high selectivity and sensitivity towards toxic Hg(II) ions compared to other tested metal ions. The binding constants (Ka) of receptors 1 and 2 are 2.53 × 105 M−1 and 0.99 × 105 M−1, respectively, which are reported with the help of Benesi–Hildebrand method.

 Please wait while we load your content...
Please wait while we load your content...