3D reticulate CoxNi3−xS2 nanostructure on nickel foam as a new type of electroactive material for high-performance supercapacitors†
Abstract
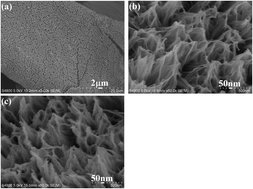
3D reticulate CoxNi3−xS2 nanostructures were grown on nickel foam using a simple precursor sulfuration route. Compared with monometallic Ni3S2, the introduction of an appropriate content of Co could markedly improve electrochemical properties. Also, the electrochemical performance of as-prepared CoxNi3−xS2 nanostructures could be tuned by the Co content added to the Ni3S2. The investigation showed that 3D CoxNi3−xS2 nanostructures prepared from the system with initial Co2+/Ni2+ molar ratio of 2/1 (labeled as S2/1) exhibited the best energy storage properties. At a current density of 2 mA cm−2, the areal capacitance of S2/1 reached 3030 mF cm−2; and even at a current density of 30 mA cm−2, the areal capacitance reached 2268 mF cm−1. After 6500 cycles at a current density of 5 mA cm−2, the capacitance retention retained 92.8% of the initial value, exhibiting excellent cycling stability. After 3D reticulate S2/1 nanostructures were fabricated into an asymmetrical supercapacitor with activated carbon, the energy density achieved 73.5 W h kg−1 at a power density of 600 W kg−1; and at high power density of 3024.06 W kg−1, the energy density could still reach 50.04 W h kg−1, which presents potential applications for energy storage as a high performance electrode material.

 Please wait while we load your content...
Please wait while we load your content...