Fabrication and characterization of bioactive glass/alginate composite scaffolds by a self-crosslinking processing for bone regeneration
Fujian Zhaoabc,
Wen Zhangabc,
Xiaoling Fuabc,
Weihan Xieabc and
Xiaofeng Chen*abc
aDepartment of Biomedical Engineering, School of Materials Science and Engineering, South China University of Technology, Guangzhou 510641, China. E-mail: chenxf@scut.edu.cn; Fax: +86 20 2223 6088; Tel: +86 20 2223 6283
bNational Engineering Research Center for Tissue Restoration and Reconstruction, Guangzhou 510006, China
cKey Laboratory of Biomedical Materials and Engineering, Ministry of Education, South China University of Technology, Guangzhou 510006, China
First published on 12th September 2016
Abstract
The objective of this study was to synthesize and characterize self-crosslinked bioactive glass/alginate composite scaffolds, as a kind of potential biomaterial for bone regeneration. The scaffolds, fabricated through a self-crosslinking process of alginate by bioactive glass microspheres provided Ca2+ completely without any organic solvent, crosslinking agent, or binder. The microstructure, mechanical properties, apatite-forming ability, ionic release, adhesion, proliferation, and ALP activity of human bone marrow-derived mesenchymal stem cells (hBMSCs) of the scaffolds were evaluated. The results showed that uniform films could be obtained on the surface as well as in abundant crosslinking bridges in the interior of the scaffolds. The rapidly released Ca2+ from bioactive glass could be temporarily stored in alginate, which achieved the controlling release. The scaffolds, with porosities of 75–57%, had a compressive strength in the range of human trabecular bone. At the same time, they presented an excellent apatite formation ability in vitro. In vitro cellular study confirmed that the alginate films temporarily separated cells from bioactive glass which promoted early cell adhesion and all the groups showed good cell proliferation and ALP activity. All these results demonstrate that self-crosslinked scaffolds represent promising candidates for bone regeneration.
Introduction
Restoring bone defects caused by trauma, injury, disease, or aging still remains a significant challenge. Although autografts are considered as the “gold standard” for bone transplantation, the drawbacks of autografts, such as graft donor site morbidity and lengthening of the procedure during the process of harvesting, limit their application in clinics.1 To solve this problem, biomaterials with the function of bone repairing are thought to be an alternative choice in the future.2Recently, a wide variety of biomaterials have been used for bone repairing, including organic and inorganic materials.3,4 Among these biomaterials, bioactive glass (BG) is a better choice due to its enhanced biocompatibility, osteoconductive and osteoinductive properties.5,6 When contacted with body fluids, ions (Si, Ca, P, etc.) can be quickly released from bioactive glass, which activates osteogenesis related signaling pathway.7,8 Since the discovery of bioactive glass by L. L Hench, it has been widely applied in clinics as bone fillers, bone repair materials, and adjuvants in bone grafts. After decades of development, bioactive glass was prepared by combining a sol–gel technique and organic template method, which could achieve precise control of size and morphology.9 Compared with conventional bioactive glasses, micro/nano bioactive glasses have superior apatite formation ability and biological activity due to their significantly increased surface area.
However, current bioactive glass products (such as NovaBone®, PerioGlas®) are mostly in the form of powder, which cannot restore the bone defects requiring certain mechanical supporting. Bioactive glass scaffolds, with pore structure and certain mechanical properties, can solve this problem.10,11 Traditional methods to fabricate such scaffolds, like polymer foam replication12 and freeze-drying,13 have limitations to control the pore structure and mechanical properties. Recently, 3D printing techniques received considerable interest to produce customized 3D porous scaffolds through computer aided design (CAD).14 This mild printing process has significant advantages such as precise control of the scaffolds architecture and reliable reproducibility.
Since bioactive glass particles cannot be connected together without high temperature sintering, a suitable binder must be selected. Previous studies had shown that bioactive glass particles could be incorporated into poly(vinyl alcohol) PVA,15 poly ε-caprolactone (PCL),16 poly(lactide-glycolide) (PLGA),17 and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx)18 to improve their mechanical stability. However, most of these materials have to be treated by a heat process or organic solvents, limiting their application for preparing excellent performance scaffolds.
Alginate is a naturally occurring anionic polymer typically obtained from various species of kelp. It has been widely used as a biomaterial for bone tissue engineering19,20 and orthopedics21 due to its biocompatibility, hydrophilicity, and biodegradability under normal physiological conditions. It can be easily dissolved in aqueous solutions at room temperature without any organic solvent. When mixed with di- or trivalent cations like Ca2+, alginate can shape a stable hydrogel, which is stacked to form an egg-box-like structure.22
Due to biocompatibility and crosslinking properties, alginates have been used in scaffolds fabrication combined with bioactive glass in some reports. For example, Mouriño et al.23 developed Bioglass®-based scaffolds coated by sodium alginate cross-linked with Ga3+ for bone tissue engineering. They reported that the presence of Ga-alginate conferred prophylaxis against infections. Erol et al.24 prepared porous bioactive glass scaffolds using a foam replica method through a sintering process. Then the porous scaffolds were dipped in alginate solution for cross-linking by adding copper ions. Most of the relevant studies need a complex preparation process, like adding crosslinking ion24–26 or binder.27 However, in our current work, self-crosslinked bioactive glass/alginate composite scaffolds were developed without using any organic solvent, crosslinking agent, or binder. The crosslinking reaction of alginate only used rapidly released Ca2+ from 3D printed bioactive glass scaffolds. Finally, the microstructure, porosities, and mechanical properties, apatite formation ability, and cell biocompatibility of the scaffolds were investigated.
Materials and methods
Materials
Absolute ethanol, tetraethyl orthosilicate (TEOS), triethylphosphate (TEP), and calcium nitrate tetrahydrate (CN) were purchased from Guangzhou Chemical Reagent Factory Co., Ltd (Guangzhou, P. R. China). Dodecylamine (DDA), methylcellulose (MC), and sodium alginate (SA) (viscosity 20–40 c.p.s., 25 °C) were supplied by Aladdin (Shanghai, P. R. China). All chemical reagents were analytical grade.Synthesis of bioactive glass microspheres (BGM)
BGM, with a molar ratio of SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CaO
CaO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2O5 = 80
P2O5 = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, were synthesized by a sol–gel co-template method according to our previous reports.28,29 In brief, 40 g DDA was dissolved in 250 mL deionized water and 800 mL ethanol. Then TEOS (160 mL), TEP (104.9 mL) and CN (242.1 g) were added in that order to the above solution over a 30 min interval with magnetic stirring at 40 °C. The resulting solution was vigorously stirred for another 3 h, and the white precipitate was collected by filtration and dried at 60 °C for 24 h. Then, the dry precipitate was calcined at 650 °C for 3 h to obtain BGM.
5, were synthesized by a sol–gel co-template method according to our previous reports.28,29 In brief, 40 g DDA was dissolved in 250 mL deionized water and 800 mL ethanol. Then TEOS (160 mL), TEP (104.9 mL) and CN (242.1 g) were added in that order to the above solution over a 30 min interval with magnetic stirring at 40 °C. The resulting solution was vigorously stirred for another 3 h, and the white precipitate was collected by filtration and dried at 60 °C for 24 h. Then, the dry precipitate was calcined at 650 °C for 3 h to obtain BGM.
Preparation of self-crosslinking scaffolds
The first step was preparation of the structural framework of BGM scaffolds by a 3D printing method. The initial paste was prepared by mixing BGM together with MC, ethanol, and deionized water in a mass ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Then it was milled for 2 h to obtain a uniform paste. The scaffolds were fabricated using a 4th generation 3D-Bioplotter system (EnvisionTEC, Germany) under the guide of supporting computer workstations. The desired scaffold models were designed by CAD. The paste was extruded through a conic plastic nozzle with an inside diameter of 400 μm with an interval between strands of 700 μm at room temperature.
5. Then it was milled for 2 h to obtain a uniform paste. The scaffolds were fabricated using a 4th generation 3D-Bioplotter system (EnvisionTEC, Germany) under the guide of supporting computer workstations. The desired scaffold models were designed by CAD. The paste was extruded through a conic plastic nozzle with an inside diameter of 400 μm with an interval between strands of 700 μm at room temperature.
After drying at room temperature, the scaffolds were heated at a relatively low temperature (300 °C) for 2 h to ensure the methylcellulose completely decomposed, shaping the structural framework of BGMS. Then, BGMS were carefully immersed in sodium alginate solution, with certain amounts of 1%, 3%, 5%, or 7%, which were marked as BGMS-1, BGMS-3, BGMS-5 and BGMS-7, respectively. Then, the scaffolds were placed under vacuum for 30 min to ensure that the sodium alginate diffused into the pores of the scaffolds. Afterwards, the scaffolds were transferred to a chamber (37 °C, 100% humidity) for 6 h without adding any external calcium for a self-crosslinking process. Finally, the self-crosslinked scaffolds were dried at 60 °C for 24 h.
Physicochemical characterization of the self-crosslinked scaffolds
Morphology of the scaffolds before and after crosslinking was characterized by scanning electron microscopy (SEM; DSM 982-Gemini, Zeiss, Oberkochen, Germany). Thermal behavior of the 3D printed scaffolds before heat treatment was investigated from room temperature up to 600 °C using a TG-DSC instrument (Netzsch STA449, Germany) at a heating rate of 10 °C min−1 under air atmosphere. The porosity of the crosslinked scaffolds were measured with a size of Φ 10 mm × h 5 mm according to Archimedes' principle in a water medium. The porosity (P) was calculated using the following formula: P = (Wsat − Wdry)/(Wsat − Wsus) × 100%, where Wdry is the dry weight of scaffolds, Wsus is the weight of scaffolds suspended in water, and Wsat is the weight of scaffolds saturated with water. The compressive strength of crosslinked scaffolds and BGMS (Φ 10 mm × h 8 mm) was determined using an electronic universal material testing machine (Instron 5967, INSTRON Co., USA). The loading velocity was 1 mm min−1 and 20% of compressive deformation was achieved for all scaffolds at room temperature. The compressive modulus were calculated by experimental data.Analysis of apatite formation ability and ionic release
Apatite formation ability of BGMS and self-crosslinked scaffolds were determined in vitro by soaking scaffolds in simulated body fluids (SBF), as described by Kokubo et al.30 The scaffolds were soaked in SBF (VSBF/Mscaffolds: 100 mL g−1) for 3 d in a polyethylene bottle at 37 °C, respectively. Then, they were collected from SBF, rinsed with distilled water 3 times, and dried at 60 °C overnight. SEM and X-ray diffraction (Bruker D8) were used to characterize the surface microtopography and apatite mineralization of self-crosslinked scaffolds.Ionic release behaviour of self-crosslinked scaffolds was studied by soaking BGMS and self-crosslinked scaffolds in SBF at 37 °C for 0.5, 1, 3, 5, and 7 d. SBF solution was not changed during this period. At certain time points, the release of calcium, silicate, and phosphate ions were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES; PS1000-AT, Leeman, USA).
In vitro cellular evaluation of the self-crosslinked scaffolds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min at 4 °C. Finally, 50 μL supernatant was mixed with 150 μL working solution according to the manufacturer's protocol (Beyotime, China). The conversion of p-nitrophenyl phosphate into p-nitrophenol in the presence of ALP was determined by measuring the absorbance at 405 nm with a microplate reader. The ALP activity was calculated from a standard curve after normalizing to the total protein content and the results were expressed in millimoles of p-nitrophenol produced per minute per milligram of protein.
000 rpm for 20 min at 4 °C. Finally, 50 μL supernatant was mixed with 150 μL working solution according to the manufacturer's protocol (Beyotime, China). The conversion of p-nitrophenyl phosphate into p-nitrophenol in the presence of ALP was determined by measuring the absorbance at 405 nm with a microplate reader. The ALP activity was calculated from a standard curve after normalizing to the total protein content and the results were expressed in millimoles of p-nitrophenol produced per minute per milligram of protein.Statistical analysis
For all experiments, the data were expressed as mean ± standard deviation (SD). The statistical analysis was carried out using one-way analysis of variance (ANOVA). A P value less than 0.05 was considered as significant.Results and discussion
Geometry and microstructure of the scaffolds
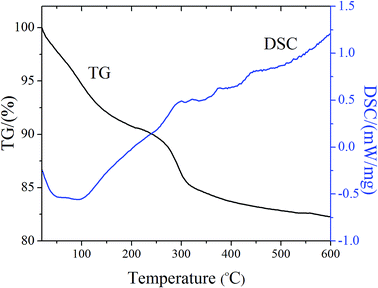
To characterize the outer shape, surface properties, and inner structure of the self-crosslinked scaffolds, SEM observations were applied with results shown in Fig. 1. It was obvious that after heat treatment (300 °C), BGMS maintained the original form (Fig. 1a1) and organics had completely disappeared (Fig. 1a2 and a3). This could be further confirmed by TG-DSC curves (Fig. 2) that a significant weight loss could be found from room temperature up to 300 °C due to dehydration and decomposition of MC from the scaffolds. However, the total weight tended to be stable from 300 °C to 600 °C, indicating the organics had completely disappeared. Compared with BGMS, after the self-crosslinking process, the scaffolds still maintained their original shape and pore size without any shrinkage and deformation, as seen from inserts in the digital photos of Fig. 1a1–e1. For the different rough surfaces of BGMS, the self-crosslinked scaffolds were covered with uniform films both on strut surface and junction areas. High magnification views of the surfaces (Fig. 1a2–e2) show that the microspheres outline of BGMS-1 can be clearly recognized, while BGMS-3, 5 and 7 gradually became blurred. This phenomenon indicated that with the increase of alginate concentration, the films presented a thickening tendency. Similar alginate cross-linked films had been prepared on boron-containing bioactive glass-based scaffolds surface by the foam replica method.24Inner structures of the scaffolds are shown in Fig. 1a3–e3. It can be observed that alginate had flowed into the interior of the scaffolds and covered the surface of the microspheres. In addition, the microspheres were connected with each other by crosslinking bridges. With an increase of alginate concentration, the number of crosslinking bridges increased gradually (as shown in Fig. 1b3–d3), except for BGMS-7 (Fig. 1e3). The reason is that high concentrations of alginate reduced the liquidity of the liquid, which was not conducive for alginate to flow into the interior of the scaffolds. In this study, the interior of self-crosslinked scaffolds could get the most alginate when the sodium alginate concentration was 5%. However, this concentration was not enough to form crosslinked films in the limited space. As a result, even though after sufficient time of crosslinking, the inner surface structure of self-crosslinked scaffolds still formed the crosslinking bridges shape rather than a films-shape.
Previous studies had prepared alginate/hydroxyapatite composite scaffolds,26,31 bioactive glass/alginate composite scaffolds,32 calcium phosphate-alginate scaffolds,27 and alginate/silica composite scaffolds,33 but they all needed to add a crosslinking agent, like CaCl2.32 In this study, the scaffolds were prepared by using bioactive glass combined with a little alginate, without using any binder or cross-linking agent. The reason can be explained by the schematic illustration as shown in Fig. 3. Bioactive glass microspheres were prepared by using dodecylamine as a template. In the process of the reaction, the –Si–O–Si– network was quickly formed through a condensation reaction, while the calcium was deposited onto silica particle surfaces during drying and thus limited the diffusion of calcium into microspheres. As a result, calcium only entered the superficial layer of the glass network during calcination.9,34 It had been reported that Ca2+ can quickly be released from bioactive glass within a few hours, when contacting with solution by this method.35 Alginate solution, when in contact with the scaffolds, flows into the interior of the scaffolds through the crevices between bioactive glass microspheres. When the released Ca2+ and alginate met in the interior of scaffolds (Fig. 3a), the crosslinking reaction occurred and then the egg-box-like structure was formed. After adequate crosslinking time, the crosslinking bridges finally formed, as shown in Fig. 3b.
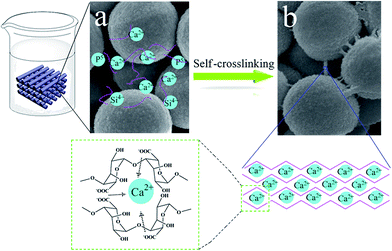 | ||
| Fig. 3 Schematic illustration of the self-crosslinking processes: (a) ions quickly released from bioactive glass, (b) formed crosslinking bridges. | ||
Porosities and mechanical properties
Porosities and compressive strengths of the self-crosslinked scaffolds are shown in Fig. 4. The porosities of scaffolds showed a slightly decrease tendency (∼75–57%), with an increase of alginate concentration. This can be explained by the SEM images of inner structures where crosslinking bridges occupied the position of the porosities as shown in Fig. 1b3–e3. Compressive strength and modulus results confirmed that the mechanical properties of self-crosslinked scaffolds were significantly improved as compared to BGMS (Fig. 4b–d). Moreover, the compressive strength and modulus of self-crosslinked scaffolds showed an increased tendency, and the mechanical property of BGMS-5 was significantly better than BGMS-1 and 3, in the compressive strength and Young's modulus (P < 0.05). The compressive strength of BGMS-5 was about 2.12 MPa, which was in the compressive strength range of human trabecular bone (ca. 2–12 MPa). A similar result was recently reported by Mishra et al. who prepared the bioactive glass–polyvinyl alcohol–alginate biocomposite materials by a surfactant foaming method, reaching a compressive strength of 1.64 MPa and elastic modulus of 18 MPa.36 | ||
| Fig. 4 (a) Porosities, (b) compressive strength, (c) typical strain–stress curves, and (d) Young's modulus of the self-crosslinked scaffolds. * indicates the significant differences, P < 0.05. | ||
In vitro ionic release and apatite formation ability
The representative morphologies and EDS compositional analysis of the scaffolds after soaking in SBF for 3 days are shown in Fig. 5. It was quite clear that flower-like apatite precipitates could be observed on the microsphere's surface of BGMS (Fig. 5a1). For BGMS-1 (Fig. 5b1) and BGMS-3 (Fig. 5c1), the films covered on the surface had completely disappeared, and the microspheres were completely exposed. It can be observed that the exposed microspheres of BGMS-1 are fully covered by flower-like apatite precipitates which aggregated together and intertwined with each other. EDS compositional analysis of BGMS-1 (Fig. 5b2) confirmed that it had a similar Ca/P molar ratio with BGMS (Fig. 5a2) which indicates a similar apatite formation ability. However, the microsphere's surfaces of BGMS-3 solely became coarser. The poor apatite formation ability could be attributed to the shorter contact time with SBF, which indicates presence of the film's hindered apatite formation. Once bioactive glasses contacted with SBF, a silica-rich layer could be formed on the surface, followed by nucleation and growth of HA crystals on top of the silica-rich layer.37,38 However, BGMS-5 (Fig. 5d1) and BGMS-7 (Fig. 5e1) had still been covered with large aggregates and entire films, respectively. The EDS results also confirmed existence of the films with high contents of C and Na, as shown in Fig. 5d2 and e2. This phenomenon could be explained by the swelling theory.39,40 When immersed in SBF, the thin films can release divalent ions into the surrounding media due to exchange reactions with monovalent cations, such as Na+.41,42The insert's digital photos shown in Fig. 5a1–a5 are the scaffolds after soaking in SBF for 3 days. It was obvious that BGMS had completely collapsed, while all the self-crosslinked scaffolds still maintained their original shapes. This can be explained that, although the thin films on the surface of scaffolds disappeared, crosslinking bridges still existed, as indicated by arrows marked in Fig. 5b1–d1. Meanwhile, the hydroxyapatite crystal layer formed on the surface further prevented breakup.
Fig. 6 shows XRD spectra of the scaffolds after being immersed in SBF for 3 days. The amorphous state of BGMS with a broad peak at 2θ = 23° confirmed that the sintering process of removing organic matter did not affect the low intensity crystalline nature of bioactive glass microspheres.43 With increasing concentration of sodium alginate, the intensities of the broad peak at 23° gradually weaken. This phenomenon could be explained by SEM facts that high concentrations of alginate could form thicker films (Fig. 1). These results are also in agreement with previous data on bioglass-derived glass ceramic scaffolds coated by alginate cross-linked with gallium.25 In addition to the characteristic broad peak of the bioactive glass, some weaker peaks were observed at 2θ = 26° (002), 32° (211), 39° (310), 46° (222) and 49° (213) in BGMS-1, 3, and 5, corresponding to the crystallinity for hydroxyapatite (HA) (JCPDS 09-0432). These phenomena are consistent with the SEM images (Fig. 5), which indicates the superior apatite formation ability of self-crosslinked scaffolds.
The ionic release in SBF of BGMS and self-crosslinked scaffolds are shown in Fig. 7. It can be seen that the changes in concentration of Ca, P, and Si ions for BGMS is similar with previous research of bioactive glass powder.35 As for self-crosslinked scaffolds, Ca concentrations of self-crosslinked scaffolds on day 1 (Fig. 7a) were lower than BGMS and followed by a slow decrease, which indicated that Ca ions showed a controlled release behavior. The P concentrations of self-crosslinked scaffolds (Fig. 7b) in SBF showed an increase at day 0.5 (except BGMS-1, which showed a slight decline) followed by a decrease during the residual period. The reason is that apatite formation in day 1 was very weak, as the thin films were not damaged, and therefore didn't consume P. However, P concentrations of BGMS-1,3 reached the same level as BGMS at day 7, indicating similar apatite formation ability. The Si concentrations of four self-crosslinked scaffolds groups (Fig. 7c) increased with more soaking time as the apatite formation process didn't consume Si ions.
 | ||
| Fig. 7 (a) Ca, (b) P, and (c) Si ion concentrations in SBF solutions after soaking BGMS and self-crosslinked scaffolds for various time periods. | ||
Cell responses on self-crosslinked scaffolds
To evaluate cell responses to self-crosslinked scaffolds, hBMSCs were cultivated on the scaffolds and their attachment, proliferation, and ALP activity were investigated. Fig. 8a–d shows SEM images of hBMSCs attached to scaffolds on day 2 of culturing. It was observed that the thin films on BGMS-1 and BGMS-3 had partially dissolved because the culture medium had the similar components with SBF. As shown in Fig. 8, both bioactive glass microspheres and alginate surfaces were able to support hBMSCs attachment as well as growth. It was obvious that the hBMSCs on alginate presented a well-extended morphology with more prominent filopodia, which indicated alginate was more suitable for cell adhesion, as shown in Fig. 8c and d. It is well-known that alginate has been widely used for cell immobilization and encapsulation due to biocompatibility and highly hydrophilic nature.33,44 However, the alkaline microenvironment of bioactive glass microspheres is not conducive to cell attachment in the early stage of contact with body fluid. In this study the thin films temporarily protected cells from the alkaline environment of bioactive glass, promoting early cell adhesion. At the same time, calcium slowly released from films without any loss.The proliferation of hBMSCs cultured on the four groups of self-crosslinked scaffolds was determined by CCK-8 assay, as shown in Fig. 8e. An increase in absorbance from day 1 to day 7 was recorded indicating that bioactive glass/alginate scaffolds supported cell proliferation. However, the proliferation rates on the BGMS-5, 7 were significantly higher than that on the BGMS-1, 3 on day 3, 7 (P < 0.05), suggesting higher cell viability and better biocompatibility of alginate thin films.
ALP activity of hBMSCs cultured on self-crosslinked scaffolds for 5 and 10 days are shown in Fig. 8f. It can be found that the BGMS-1 showed significantly higher ALP activity compared to BGMS-5,7 at day 5 (P < 0.05). However, there were no significant differences in ALP activity of hBMSCs on the four groups of self-crosslinked scaffolds at day 10. Some reports had confirmed that bioactive glass could influence osteoblastic cell differentiation with an increase in the level of differentiation markers like alkaline phosphatase (ALP), osteocalcin, and osteopontin.45 In this study, the lower ALP activity of BGMS-5,7 could be attributed to the thin films which temporarily hindered hBMSCs from contacting with bioactive glass (Fig. 8).
Conclusions
In the present study, self-crosslinked bioactive glass/alginate composite scaffolds were successfully fabricated by a self-crosslinking process, without using any organic solvent, crosslinking agent, or binder. The crosslinking process of alginate happened in 3D printed bioactive glass scaffolds, which were fabricated through a sintering process, and Ca2+ was completely provided by bioactive glass microspheres. The scaffolds can maintain their shape in SBF through the crosslinked bridges. Calcium ions can be temporarily stored in alginate to achieve controlling release. The scaffolds, with porosities of ∼75–57%, obtained a compressive strength in the range of human trabecular bone. At the same time, they presented a relatively excellent apatite formation ability in vitro. The biocompatible alginate films temporarily protected cells from the alkaline environment stage, which could promoted early cell adhesion. All these results suggest that the self-crosslinked bioactive glass/alginate scaffolds have the potential for bone regeneration.Acknowledgements
This study was financially supported by the Joint Funds of the National Natural Science Foundation of China (grant no. U1501245), the Fundamental Research Funds for the Central Universities (grant no. 2015ZP020, 2014ZB0018) and the National Natural Science Foundation of China (grant no. 51402108).Notes and references
- G. William, T. A. Einhorn, K. Koval, M. McKee, W. Smith, R. Sanders and T. Watson, J. Bone Jt. Surg., 2007, 89, 649–658 CrossRef PubMed.
- L. L. Hench and I. Thompson, J. R. Soc., Interface, 2010, 7, S379–S391 CrossRef CAS PubMed.
- V. Guarino, F. Causa and L. Ambrosio, Expert Rev. Med. Devices, 2007, 4, 405–418 CrossRef CAS PubMed.
- D. W. Hutmacher, J. T. Schantz, C. X. F. Lam, K. C. Tan and T. C. Lim, J. Tissue Eng. Regener. Med., 2007, 1, 245–260 CrossRef CAS PubMed.
- M. Peter, P. T. S. Kumar, N. S. Binulal, S. V. Nair, H. Tamura and R. Jayakumar, Carbohydr. Polym., 2009, 78, 926–931 CrossRef CAS.
- H. Yuan, J. D. de Bruijn, X. Zhang, C. A. van Blitterswijk and K. de Groot, J. Biomed. Mater. Res., 2001, 58, 270–276 CrossRef CAS PubMed.
- G. Jell and M. M. Stevens, J. Mater. Sci.: Mater. Med., 2006, 17, 997–1002 CrossRef CAS PubMed.
- I. D. Xynos, A. J. Edgar, L. D. Buttery, L. L. Hench and J. M. Polak, J. Biomed. Mater. Res., 2001, 55, 151–157 CrossRef CAS PubMed.
- S. Labbaf, O. Tsigkou, K. H. Müller, M. M. Stevens, A. E. Porter and J. R. Jones, Biomaterials, 2011, 32, 1010–1018 CrossRef CAS PubMed.
- L.-C. Gerhardt and A. R. Boccaccini, Materials, 2010, 3, 3867–3910 CrossRef CAS.
- Q. Fu, E. Saiz, M. N. Rahaman and A. P. Tomsia, Mater. Sci. Eng., C, 2011, 31, 1245–1256 CrossRef CAS PubMed.
- C. Vitale-Brovarone, E. Verné, L. Robiglio, P. Appendino, F. Bassi, G. Martinasso, G. Muzio and R. Canuto, Acta Biomater., 2007, 3, 199–208 CrossRef CAS PubMed.
- Q. Fu, M. N. Rahaman, B. S. Bal and R. F. Brown, J. Biomed. Mater. Res., Part A, 2010, 93, 1380–1390 CrossRef PubMed.
- A. Gloria, T. Russo, R. De Santis and L. Ambrosio, J. Appl. Biomater. Biomech., 2009, 7, 141–152 CAS.
- J. Zhang, S. Zhao, Y. Zhu, Y. Huang, M. Zhu, C. Tao and C. Zhang, Acta Biomater., 2014, 10, 2269–2281 CrossRef CAS PubMed.
- J. Zhang, S. Zhao, M. Zhu, Y. Zhu, Y. Zhang, Z. Liu and C. Zhang, J. Mater. Chem. B, 2014, 2, 7583–7595 RSC.
- E. Pamula, J. Kokoszka, K. Cholewa-Kowalska, M. Laczka, L. Kantor, L. Niedzwiedzki, G. C. Reilly, J. Filipowska, W. Madej and M. Kolodziejczyk, Ann. Biomed. Eng., 2011, 39, 2114–2129 CrossRef PubMed.
- S. Zhao, M. Zhu, J. Zhang, Y. Zhang, Z. Liu, Y. Zhu and C. Zhang, J. Mater. Chem. B, 2014, 2, 6106–6118 RSC.
- J. Venkatesan, I. Bhatnagar, P. Manivasagan, K.-H. Kang and S.-K. Kim, Int. J. Biol. Macromol., 2015, 72, 269–281 CrossRef CAS PubMed.
- A. Gutowska, B. Jeong and M. Jasionowski, Anat. Rec., 2001, 263, 342–349 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- G. T. Grant, E. R. Morris, D. A. Rees, P. J. Smith and D. Thom, FEBS Lett., 1973, 32, 195–198 CrossRef CAS.
- V. Mouriño, P. Newby, F. Pishbin, J. Cattalini, S. Lucangioli and A. Boccaccini, Soft Matter, 2011, 7, 6705–6712 RSC.
- M. Erol, V. Mouriňo, P. Newby, X. Chatzistavrou, J. Roether, L. Hupa and A. R. Boccaccini, Acta Biomater., 2012, 8, 792–801 CrossRef CAS PubMed.
- V. Mourino, P. Newby and A. R. Boccaccini, Adv. Eng. Mater., 2010, 12, B283–B291 CrossRef.
- H. R. Lin and Y. J. Yeh, J. Biomed. Mater. Res., Part B, 2004, 71, 52–65 CrossRef PubMed.
- Y. Luo, A. Lode, F. Sonntag, B. Nies and M. Gelinsky, J. Mater. Chem. B, 2013, 1, 4088–4098 RSC.
- Q. Hu, X. Chen, N. Zhao and Y. Li, Mater. Lett., 2013, 106, 452–455 CrossRef CAS.
- Q. Hu, Y. Li, G. Miao, N. Zhao and X. Chen, RSC Adv., 2014, 4, 22678–22687 RSC.
- T. Kokubo and H. Takadama, Biomaterials, 2006, 27, 2907–2915 CrossRef CAS PubMed.
- Y. Luo, A. Lode, C. Wu, J. Chang and M. Gelinsky, ACS Appl. Mater. Interfaces, 2015, 7, 6541–6549 CAS.
- Y. Luo, C. Wu, A. Lode and M. Gelinsky, Biofabrication, 2013, 5, 015005 CrossRef PubMed.
- U. Schloßmacher, H. C. Schröder, X. Wang, Q. Feng, B. Diehl-Seifert, S. Neumann, A. Trautwein and W. E. Müller, RSC Adv., 2013, 3, 11185–11194 RSC.
- S. Lin, C. Ionescu, K. J. Pike, M. E. Smith and J. R. Jones, J. Mater. Chem., 2009, 19, 1276–1282 RSC.
- D. Arcos, A. Lopez-Noriega, E. Ruiz-Hernandez, O. Terasaki and M. Vallet-Regi, Chem. Mater., 2009, 21, 1000–1009 CrossRef CAS.
- R. Mishra, B. Basu and A. Kumar, J. Mater. Sci.: Mater. Med., 2009, 20, 2493–2500 CrossRef CAS PubMed.
- L. L. Hench, J. Biomed. Mater. Res., 1998, 41, 511–518 CrossRef CAS PubMed.
- L. L. Hench and J. West, Annu. Rev. Mater. Sci., 1995, 25, 37–68 CrossRef CAS.
- C. K. Kuo and P. X. Ma, Biomaterials, 2001, 22, 511–521 CrossRef CAS PubMed.
- C. K. Kuo and P. X. Ma, J. Biomed. Mater. Res., Part A, 2008, 84, 899–907 CrossRef PubMed.
- C. A. Bonino, M. D. Krebs, C. D. Saquing, S. I. Jeong, K. L. Shearer, E. Alsberg and S. A. Khan, Carbohydr. Polym., 2011, 85, 111–119 CrossRef CAS.
- N. Mohan and P. D. Nair, Trends Biomater. Artif. Organs, 2005, 18, 219–224 Search PubMed.
- W. Xia and J. Chang, Mater. Lett., 2007, 61, 3251–3253 CrossRef CAS.
- S. Sugiura, T. Oda, Y. Izumida, Y. Aoyagi, M. Satake, A. Ochiai, N. Ohkohchi and M. Nakajima, Biomaterials, 2005, 26, 3327–3331 CrossRef CAS PubMed.
- P. Valerio, M. M. Pereira, A. M. Goes and M. F. Leite, Biomaterials, 2004, 25, 2941–2948 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |