A functional oligonucleotide probe from an encapsulated silver nanocluster assembled by rolling circle amplification and its application in label-free sensors†
Abstract
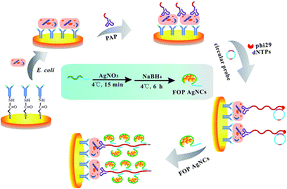
A novel label-free, low cost electrochemical biosensor for highly sensitive and selective detection of E. coli has been developed based on rolling circle amplification (RCA) coupled silver nanoclusters (AgNCs) as an effective electrochemical probe. This strategy is to our knowledge the first approach where the combination of RCA with the efficient catalytic property of AgNCs has been applied in bioanalysis. Our biosensor relies on sandwich type immuno-recognition and an in situ RCA reaction, which results in the enrichment of functional oligonucleotide probe (FOP) encapsulated AgNCs. Due to the high electrocatalytic activity of AgNCs toward H2O2 reduction, stable and sensitive electrochemical signals can be achieved from the biological recognition events. The results reveal the calibration plot obtained for E. coli O157:H7 is approximately linear from 3.7 × 10 to 3.7 × 106 cfu mL−1 with a limit of detection of 31 cfu mL−1. This label-free biosensor has advantages over conventional electrochemical assays in its significantly improved sensitivity, remarkably widened dynamic range, and substantially lowered background signal. Moreover, the proposed biosensor has been demonstrated to allow accurate quantitative assay of E. coli O157:H7 in milk samples. By virtue of these merits, our RCA coupled AgNCs-based electrochemical biosensor might create a useful and valuable tool for the detection of E. coli O157:H7 and related molecular diagnostics in food safety analysis.

 Please wait while we load your content...
Please wait while we load your content...