Efficient and green approach for the complete deprotection of O-acetylated biomolecules
Anthony Dunne and
Jose M. Palomo*
Departamento de Biocatálisis, Instituto de Catálisis (CSIC), Marie Curie 2, Cantoblanco, Campus UAM, 28049 Madrid, Spain. E-mail: josempalomo@icp.csic.es; Fax: +34 915854760
First published on 13th September 2016
Abstract
A simple, efficient and mild strategy for the complete O-deacetylation of different per-acetylated biomolecules in aqueous media has been described. Different lipases were tested but only the commercial Amano lipase A from Aspergillus niger catalyzed the complete deprotection of peracetylated α-glucose to glucose in excellent yield. The experimental conditions were tested, in particular the pH effect. The reaction was performed at different pHs considering the only enzymatic process was evaluated at pH 5 and the combination of enzymatic and chemical migration process was evaluated at higher pHs. Finally pH 7 and 25 °C were selected as best conditions. Thus this lipase fully hydrolyzed different peracetylated α-glycopyranosides (glucose, mannose, glucal, galactal) with >99% yields, whereas very good deprotecting yields (75–80%) were achieved for different acetylated β-glycopyranosides (galactose, ribofuranose) under these mild conditions. This strategy was successfully extended to the fully O-selective deprotection of acetylated nucleosides where >99% yield was rapidly obtained. No selectivity was observed for the N-deacetylation in amino acids and peptides.
Introduction
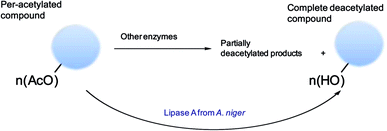
Deprotection/protection strategies have been very useful in synthetic chemistry and also are important for biotechnological industrial application.1–8 Deacetylation processes are quite important in organic synthesis, with interesting applications in pharmaceuticals preparation and production of high-added value compounds from biomass.9–12 Also this deprotection strategy is relevant in different biological processes (Fig. 1).13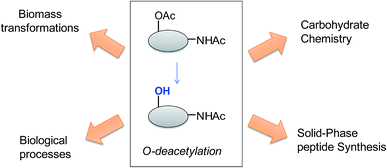 | ||
| Fig. 1 General scheme of the different applications of the O-deacetylation process in chemistry and biotechnology. | ||
The deacetylation of hydroxyl groups has been widely used in carbohydrate chemistry.11,12 Per-O-acetylated glycopyranosides and glycoderivatives are useful intermediate molecules in the synthesis of final target products – deacetylated molecules – with interesting biological activities.14–16 Also the acetyl groups combined with other protecting groups such as hydroxyls are extensively used in carbohydrate synthesis.11,12 In particular sustainable methodologies to get a complete and specific deprotection process in glycosidic moieties of glycoderivatives, such as complex oligosaccharides, glycopeptides or glycolipidated peptides, on solid-phase need to be developed.17–21
The impact of deacetylation for example of lignocellulosic materials in improved of monomeric sugar yields from natural waste components of agriculture as value-added products is of great relevance to preserve environment and biodiversity, avoiding consumption of natural resources, being precursors of interesting compounds in biofuels production industry.22
Different chemical approaches have been used in the removal of acetyl groups.23–25 However, sometimes the deacetylation is not complete, others are not completely selective or specific between O or N-deprotection, affording both O- and N-deacetylated derivatives, or not compatible with some chemical groups in the molecules to be deprotected (e.g. glycosylated aminoacids or prenylated molecules).26 For that reason, the development of green and mild strategies of deprotection is interesting in chemistry.
Several biocatalytic strategies have been developed although in most cases uncompleted deacetylation – generating mixtures of different partially unprotected products – have been found.27–32 This is especially important when using peracetylated-mono or disaccharides for the multiple acetyl groups with similar reactivity in the molecule.
Another aspect to consider in the development of an appropriate strategy is the selectivity. The adequate biocatalyst would be highly selective between O- and N-acetylation, being possible the deprotection of only one of them in molecules containing both N- and O-acetyl groups.32,33
Therefore, here we present the use of a lipase A from Aspergillus niger (A-ANL) at neutral pH and room temperature to complete deprotection of different per-acetylated biomolecules (different glycopyranosides, nucleosides) (Fig. 2) showing a very high efficient to produce the free unprotected molecule in almost all cases, and high specificity to the O-deprotection instead of the N-deprotection.
Results and discussion
Three catalysts, Novozyme435, RM-esterase (esterase purified from commercial extract of R. miehei lipase) and A-ANL, were tested by reacting them with peracetylated glucose 1 to find the most suitable catalyst to complete deprotection of fully acetyl groups. The hydrolysis using Novozyme435 and AE-esterase was tested at different pHs, evaluating the effect only of the enzyme (pH 5) and also the combination of enzymatic and chemical acyl-migration (pH 7 and 8.5) (Table 1).| Catalyst | pH | Peracetylated disappearance time (h) | Time (h) | Glucose yield (%) |
|---|---|---|---|---|
| Novozyme435 | 5.0 | 95 | 240 | 84 |
| Novozyme435 | 7.0 | 94 | 117 | 86 |
| Novozyme435 | 8.5 | 71 | 95 | 88 |
| RM-esterase | 5.0 | 167 (not fully disappeared) | 168 | 38 |
| RM-esterase | 7.0 | 168 | 168 | 40 |
| RM-esterase | 8.5 | 167 (not fully disappeared) | 167 | 55 |
| A-ANL | 7.0 | 1 | 22 | >99 |
Novozyme435 catalyzed the reaction at pH 5, producing the full disappeared of 1 after 96 h, whereas the different partially deprotected products were being transformed in the final glucose in 86% yield after 240 h. The reaction was performed at pH 7 and similar final yield of glucose was obtained although in less time (117 h) which indicated that the acyl-migration process presented at these conditions helped in the final yield. The yield was slightly higher in glucose production at similar times after incubation at pH 8.5 (Table 1). When RM-esterase was used the results were worse. After 168 h, the peracetylaed 1 spot did not completely disappear at pH 5 or to pH 8.5. At this point only around 40–50% of glucose was obtained with a mixture of partially acetylated products. In the case of pH 7 the result was similar although 1 had disappeared (Table 1). Therefore, these results demonstrated that both enzymes showed a partial selectivity and they cannot get a complete removal of all acetyl groups of the molecules.
Therefore, these results demonstrated that both enzymes showed a partial selectivity and they cannot get a complete removal of all acetyl groups of the molecules. pH 7 condition was selected at the best choice for the hydrolytic reaction.
In this way the reaction was attempted using the lipase A from A. niger (A-ANL), an enzyme which we have observed a certain low regioselectivity in some cases which is crucial in this case.34 The A-ANL catalyst was very fast in the hydrolysis, in 1 h the 1 spot in TLC had disappeared and a fully deprotection with the formation of an unique product, glucose, was obtained in >99% yield after 22 h incubation at pH 7 and room temperature (Table 1).
In other to expand the scope of the strategy with this excellent fully deprotection process, A-ANL was used as catalysts in the hydrolysis of six different glycopyranosides at the same conditions (Table 2). The results were excellent tested with two different epimeric form of glucose. When the 4-OH group was in axial position, peracetylated galactose 2, the complete deprotection was achieved after 30 h in >99% yield, whereas in the case of peracetylated mannose 3 (the 2-OH in axial position) the complete deprotection was obtained after 46 h incubation. In these three cases the enzyme was excellent to produce the free sugar although between 1.3–2 times faster in the case of glucose. However, the best result in term of activity of A-ANL in the complete hydrolysis was obtained in the case of peracetylated glycals (4 and 5). In both cases, complete deprotection was obtained after 1 h incubation in both cases peracetylated glucal 4 and peracetylated galactal 5. The structural differences in the glycals (preferred half-chair conformation) against sugar (typical chair conformation) makes easier the location of the compound in the active site and could be explain why obtaining similar results faster with these molecules.10
Also, for more complex sugar scheme, the beta-glycopyranosides has been described a very difficult molecules for lipase hydrolysis. However, A-ANL catalyzed the complete deprotection of β-galactose pentaacetate 6 and β-ribofuranose 7 in high yields, 80% and 75% respectively.
Therefore, these demonstrated the extraordinary capacity of this enzyme at these mild conditions for the deacetylation of different kind of glycopyranosides.
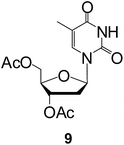
To evaluate also the capacity in other kind of molecules, the enzymatic process was studied using per-acetylated nucleosides, adenosine (8) and thymidine (9) (Table 3). The deprotection of both was very fast and a full O-deprotected adenosine was obtained after 30 min incubation. Also, the same test using a fully acetylated adenosine OH in the sugar part and the amino in the base, showed the O-selectivity of the enzyme, the N-acetylated group in adenosine was not removed (data not shown). The complete hydrolysis of di-acetylated thymidine was obtained in 2 h.
The test of the non selectivity against N-acetyl groups was confirmed by incubation of the enzyme with the different aminoacids and peptides (10–12). In all cases no reaction was observed after 50 h incubation (Table 3).
Conclusion
Here a very mild, versatile and efficient strategy for the complete deprotection of different kind of per-acetylated molecules (from pentacetylated to monoacetylated ones) has been successfully performed using the commercial enzyme, lipase A from Aspergillus niger. Excellent yields for the complete acetyl groups removal have been obtained at very mild conditions, neutral pH and room temperature. This enzyme showed a high selectivity to the O-deacetylation instead of the N-acetylation. This strategy does not require of special equipment and can be performed in any laboratory. Interesting applications of it could be in solid-phase peptide synthesis of glycopeptides and oligosaccharides and also as complementary enzyme to cellulases in the production of high added values such as xylose or xylooligosaccharides from agricultural sources.Experimental
Materials
Lipase A from Aspergillus niger (A-ANL), per-O-acetylated α-glucose (1), per-O-acetylated α-galactose (2), per-O-acetylated mannose (3), per-O-acetylated glucal (4), per-O-acetylated galactal (5), per-O-acetylated β-galactose (6), per-O-acetylated-β-ribofuranose (7), glucose, galactose, mannose, ribofuranose, galactal, glucal, thymidine, adenosine, Ac-NH-Lys-COOH (10) and Ac-NH-Tyr-COOH (11) were from Sigma Chem. Co (St. Louis, USA). The immobilized CAL-B (Novozyme435) and the lipase from Rhizomucor miehei (RML) were kindly donated by Novozymes (Denmark). Octyl-Sepharose was purchased from Sepharose Bead Technologies. RM-esterase, a contaminant esterase presents in the commercial extracted of RML, was purified as previously described.35 Ac-NH-Cys-Phe-Phe-CONH2 (12) was from INBIOS (Italy). TLC analyses were run on silica plates (Merck 60 F254).Synthesis of per-O-acetylated nucleosides
A suspension of adenosine or thymidine (12 mmol) in acetonitrile (1 mL/0.2 mmol of nucleoside) was treated with triethylamine (TEA, 4 equiv.) and acetic anhydride (4 equiv.) in the presence of a catalytic amount of 4-(dimethylamino)pyridine (DMAP). The resulting mixture was stirred at room temperature until the reaction was complete (TLC analysis) and was then diluted with chloroform and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The organic phase was separated, washed with water (4 × 20–50 mL) and dried (Na2SO4), filtered, and concentrated in vacuo. The residue was purified by silica gel column chromatography (CH2Cl2 100% to CH2Cl2/MeOH, 98
1). The organic phase was separated, washed with water (4 × 20–50 mL) and dried (Na2SO4), filtered, and concentrated in vacuo. The residue was purified by silica gel column chromatography (CH2Cl2 100% to CH2Cl2/MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford the 2′,3′,5′-tri-O-acetyladenosine 8 or 3′,5′-di-O-acetylthymidine 9 (88%). TLC plates were eluted using ethyl acetate/hexane (7/3) for 9 (Rf = 0.32) and dichloromethane/methanol (9/1) for 8 (Rf = 0.65) and visualized by UV light (254 nm). NMR data are in agreement with the reported values.36,37
2) to afford the 2′,3′,5′-tri-O-acetyladenosine 8 or 3′,5′-di-O-acetylthymidine 9 (88%). TLC plates were eluted using ethyl acetate/hexane (7/3) for 9 (Rf = 0.32) and dichloromethane/methanol (9/1) for 8 (Rf = 0.65) and visualized by UV light (254 nm). NMR data are in agreement with the reported values.36,37
Biocatalytic deprotection of per-O-acetylated glucose (1) at different pHs
Per-O-acetylated α-glucose (1) (50 mg, 10 mM) was dissolved in 1.3 mL of acetonitrile. This solution was mixture with 4.5 mL of 100 mM phosphate buffer at different pHs (5, 7 or 8.5). 3 g of Novozyme435, 0.5 g of RM-esterase or 0.5 g of A-ANL were added. The reaction was followed by TLC analysis, using as eluent hexane/ethyl acetate (1/1) for 1 (Rf = 0.56) and acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (8.5/1.5) for glucose (Rf = 0.40). TLC were staining by soaking in a solution of 5% H2SO4, in methanol and then burn using a hot plate to produce the spots. Free commercial available glucose was used as standard.
water (8.5/1.5) for glucose (Rf = 0.40). TLC were staining by soaking in a solution of 5% H2SO4, in methanol and then burn using a hot plate to produce the spots. Free commercial available glucose was used as standard.
Hydrolytic deprotection of per-O-acetylated glycopyranosides and nucleosides catalyzed by A. niger lipase
Different per-O-acetylated glycopyranosides (2–7) and nucleosides (8, 9) were dissolved at 10 mM concentration in a 5.5 mL solution (4.5 mL of 100 mM phosphate buffer at pH 7 and 1 mL of acetonitrile). 0.1 g of A-ANL powder was added to 1 mL of this solution in each case and the reaction was followed by TLC at different times. The TLC conditions used were the same previously described acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (8.5/1.5) as eluent and staining by soaking in a solution of 5% H2SO4, in methanol and then burn using a hot plate to produce the spots. At this conditions, Rf = 0.40 for galactose, mannose, Rf = 0.75 for glucal and galactal, and Rf = 0.5 for ribofuranose. Nucleosides were followed as previously described and visualized by UV light (254 nm). The different free commercial available glycopyranosides and nucleosides were used as standards.
water (8.5/1.5) as eluent and staining by soaking in a solution of 5% H2SO4, in methanol and then burn using a hot plate to produce the spots. At this conditions, Rf = 0.40 for galactose, mannose, Rf = 0.75 for glucal and galactal, and Rf = 0.5 for ribofuranose. Nucleosides were followed as previously described and visualized by UV light (254 nm). The different free commercial available glycopyranosides and nucleosides were used as standards.
Hydrolytic deprotection of acetylated aminoacids and peptides catalyzed by A. niger lipase
Acetylated products 10–12 were dissolved at 10 mM concentration in a 5.5 mL solution (4.5 mL of 100 mM phosphate buffer at pH 7 and 1 mL of acetonitrile). 0.1 g of A-ANL powder was added to 1 mL of this solution in each case and the reaction was followed by TLC at different times. TLC plate of 10 was eluted in n-butanol/acetic acid/water (3/1/1) (Rf of 10 = 0.80) and stained using bromocresol. For compound 11 TLC plates were eluted in a mobile phase of acetonitrile/water/acetic acid (8.5/1.4/0.2) and visualized by UV light (Rf of 11 = 0.50). In the case of peptide 12 the eluent was acetonitrile/dichloromethane (8/2) and TLC spot was visualized by UV light (Rf of 12 = 0.80).Acknowledgements
The authors thank the Ramon Areces Foundation for financial support and the European community for an ERA HEI mobility grant to Anthony Dunne. We also thank the support by The Spanish National Research Council (CSIC) and Dr Martinez from Novozymes for the generous gift of lipases.References
- M. J. Hansen, W. A. Velema, M. M. Lerch, W. Szymanski and B. L. Feringa, Chem. Soc. Rev., 2015, 44, 3358–3377 RSC.
- R. M. N. Kalla, M. R. Kim, Y. N. Kim and I. Kim, New J. Chem., 2016, 40, 687–693 RSC.
- X. Wu, L. Su, G. Chen and M. Jiang, Macromolecules, 2015, 48, 3705–3712 CrossRef CAS.
- P. G. M. Wuts, Greene's Protective Groups in Organic Synthesis, 5th edn, 2014, pp. 1–1360 Search PubMed.
- C. M. Grison, A. Velati, V. Escande and C. Grison, Environ. Sci. Pollut. Res., 2015, 22, 5686–5698 CrossRef CAS PubMed.
- S. Das, R. Borah, R. R. Devi and A. J. Thakur, Molecular iodine in protection and deprotection chemistry, Synlett, 2008, 18, 2741–2762 Search PubMed.
- R. S. Nandurdikar, L. K. Keefer and J. E. Saavedra, Chem. Commun., 2011, 47, 6710–6712 RSC.
- V. K. Das, S. Das and A. J. Thakur, Green Chem. Lett. Rev., 2012, 5, 577–586 CrossRef CAS.
- P. J. Moynihan, D. Sychantha and A. J. Clarke, Bioorg. Chem., 2014, 54, 44–50 CrossRef CAS PubMed.
- M. Filice, J. M. Guisan, M. Terreni and J. M. Palomo, Nat. Protoc., 2012, 7, 1783–1796 CrossRef CAS PubMed.
- D. Ye, G. Deng, W. Liu, Y. Zhou, E. Feng, H. Jiang and H. Liu, Tetrahedron, 2008, 64, 6544–6550 CrossRef CAS.
- O. Seitz and C.-H. Wong, J. Am. Chem. Soc., 1997, 119, 8766–8776 CrossRef CAS.
- Y. Mizuguchi, S. Specht, J. G. Lunz, K. Isse, N. Corbitt, T. Takizawa and A. J. Demetris, BMC Mol. Biol., 2012, 13, 20 CrossRef CAS PubMed.
- L. Zhao, M. Wu, C. Xiao, L. Yang, L. Zhou, N. Gaoa, Z. Li, J. Chen, J. Chen, J. Liu, H. Qin and J. Zhao, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 8284–8289 CrossRef CAS PubMed.
- E. Andrés, D. Albesa-Jové, X. Biarnés, B. M. Moerschbacher, M. E. Guerin and A. Planas, Angew. Chem., Int. Ed., 2014, 53, 6882–6887 CrossRef PubMed.
- S. Han, T. Kanamoto, H. Nakashima and T. Yoshida, Carbohydr. Polym., 2012, 90, 1061–1068 CrossRef CAS PubMed.
- C. S. Bennett, Org. Biomol. Chem., 2014, 12, 1686–1698 CAS.
- M. M. L. Zulueta, S.-Y. Lin, Y.-P. Hu and S.-C. Hung, Curr. Opin. Chem. Biol., 2013, 17, 1023–1029 CrossRef CAS PubMed.
- C.-H. Hsu, S.-C. Hung, C.-Y. Wu and C.-H. Wong, Angew. Chem., Int. Ed., 2011, 50, 11872–11923 CrossRef CAS PubMed.
- M. Liu, G. Barany and D. Live, Carbohydr. Res., 2005, 340, 2111–2122 CrossRef CAS PubMed.
- E. M. Jones and R. Polt, Front. Chem., 2015, 3, 40 Search PubMed.
- X. Chen, J. Shekiro, M. A. Franden, W. Wang, M. Zhang, E. Kuhn, D. K. Johnson and M. P. Tucker, Biotechnol. Biofuels, 2012, 5, 8 CrossRef CAS PubMed.
- R. Khan, P. A. Konowicz, L. Gardossi, M. Matulová and S. De Gennaro, Aust. J. Chem., 1996, 49, 293–298 CrossRef CAS.
- M. Thomas, F. Rivault, I. Tranoy-Opalinski, J. Roche, J.-P. Gesson and S. Papot, Bioorg. Med. Chem. Lett., 2007, 17, 983–986 CrossRef CAS PubMed.
- Carbohydrates in Chemistry and Biology, ed. B. Ernst, G. W. Hart and P. Sinay, Wiley-VCH, 2000, vol. 1 Search PubMed.
- J. M. Palomo, M. Lumbierres and H. Waldmann, Angew. Chem., Int. Ed., 2006, 45, 477–481 CrossRef CAS PubMed.
- Y. Zhang, X. Mu, H. Wang, B. Li and H. Peng, J. Agric. Food Chem., 2014, 62, 4661–4667 CrossRef CAS PubMed.
- P. D. Beaney, Q. Gan, T. R. A. Magee, M. Healy and J. Lizardi-Mendoza, J. Chem. Technol. Biotechnol., 2007, 82, 165–173 CrossRef CAS.
- N. Pareek, V. Vivekanand, P. Agarwal, S. Saroj and R. P. Singh, Carbohydr. Polym., 2013, 96, 417–425 CrossRef CAS PubMed.
- B.-D. Ma, H.-L. Yu, J. Pan, J.-Y. Liu, X. Ju and J.-H. Xu, Bioresour. Technol., 2013, 133, 354–360 CrossRef CAS PubMed.
- P. Biely, Biotechnol. Adv., 2012, 30, 1575–1588 CrossRef CAS PubMed.
- A. Baba and T. Yoshioka, J. Org. Chem., 2012, 77, 1675–1684 CrossRef CAS PubMed.
- V. Ferrari, M. Serpi, C. McGuigan and F. Pertusati, Nucleosides, Nucleotides Nucleic Acids, 2015, 34, 799–814 CAS.
- D. S. Rodrigues, A. A. Mendes, M. Filice, J. M. Guisan, R. Fernandez-Lafuente and J. M. Palomo, J. Mol. Catal. B: Enzym., 2009, 58, 36–40 CrossRef CAS.
- I. Nieto, S. Rocchietti, D. Ubiali, G. Speranza, C. F. Morelli, I. E. Fuentes, A. R. Alcantara and M. Terreni, Enzyme Microb. Technol., 2005, 37, 514–520 CrossRef CAS.
- O. Romero, M. Filice, B. De Las Rivas, C. Carrasco-Lopez, J. Klett, A. Morreale, J. A. Hermoso, J. M. Guisan, O. Abian and J. M. Palomo, Chem. Commun., 2012, 48, 9053–9055 RSC.
- T. Bavaro, S. Rocchietti, D. Ubiali, M. Filice, M. Terreni and M. Pregnolato, Eur. J. Org. Chem., 2009, 12, 1967–1975 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |