Optimized synthesis of CuInS2/ZnS:Al–TiO2 nanocomposites for 1,3-dichloropropene photodegradation
Abstract
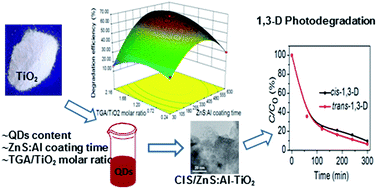
A novel and improved protocol was developed to prepare CuInS2/ZnS:Al-sensitized TiO2 (CIS/ZnS:Al–TiO2) nanocomposites for 1,3-dichloropropene (1,3-D) photodegradation. Response surface methodology was employed to model and optimize the operational parameters of this protocol. The CIS/ZnS:Al quantum dot (QDs) content, ZnS:Al coating time, and TGA/TiO2 molar ratio were chosen as independent variables at three levels by using the Box–Behnken design; their combined effects on the 1,3-D degradation efficiency were investigated. The synthesized CIS/ZnS:Al QDs and CIS/ZnS:Al–TiO2 nanocomposites were characterized by their photoluminescence intensity, UV-vis absorption spectra, X-ray diffraction, and transmission electron microscopy. Based on the experimental results, an empirical expression was established and subsequently applied to predict the 1,3-D degradation efficiency with the prepared photocatalysts. The predicted degradation efficiencies matched well with the experimental values (R2, 0.9874). The ANOVA results showed that the significance of the parameters was ZnS:Al coating time > QDs content > TGA/TiO2 molar ratio. The 3D response surface plots indicated that the optimum synthesis parameters were a CIS/ZnS:Al QDs content of 23%, a coating time of 418 min, and a molar ratio of 1.6. The 1,3-D degradation efficiency with the optimized photocatalyst reached 92% under an irradiation time of 5 h. The photodegradation kinetics of 1,3-D was well fitted with a pseudo-first-order kinetic equation. The experimental design and theoretical prediction methods in this work are of great significance in the design and development of high-performance CIS/ZnS:Al–TiO2 nanocomposites.

 Please wait while we load your content...
Please wait while we load your content...