One-step synthesis of recycled 3D CeVO4/rGO composite aerogels for efficient degradation of organic dyes†
Q. Q. Liu*,
C. Y. Fan,
H. Tang,
T. D. Ma and
J. Y. Shen
School of Materials Science and Engineering, Jiangsu University, 301 Xuefu Road, Zhenjiang, Jiangsu 212013, PR China. E-mail: liu_qin_qin@126.com
First published on 1st September 2016
Abstract
Three dimensional (3D) CeVO4/rGO porous aerogels were fabricated by a one-pot hydrothermal method. The as-prepared aerogels were characterized by X-ray diffraction (XRD), Raman spectroscopy, field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), UV-visible spectroscopy, electrochemical impedance spectroscopy (EIS) and fluorescence spectroscopy (FL) techniques. The results indicated that these aerogels showed a 3D architecture consisting of crosslinked rGO sheets in which CeVO4 particles were fully embedded. The specific surface area of the hybrid aerogel (143.2 m2 g−1) was demonstrated to be 24.3 times higher than that of bare CeVO4. The photocatalytic activities of the aerogels were investigated through the degradation of methylene blue under visible light irradiation. In comparison with the bare CeVO4 particles, the CeVO4/rGO aerogels showed significantly improved photocatalytic efficiency. The enhanced photocatalytic mechanism was mainly due to the synergistic effect between the larger surface area, porous structure of the aerogel and the effective separation of the photo-generated electron–hole pairs. The 3D block structure of lightweight CeVO4/rGO porous aerogels could not only contribute to their easy separation from the dispersion after photocatalysis, and also to their reuse for practical application.
1. Introduction
As an important aspect of environmental pollution, water pollution is severely threatening people's health. For instance, organic dyes are often disposed of in wastewater discharged into the local environment without sewage treatment. Photocatalytic degradation has been proved as a clean tool for purifying wastewater1–3 or CO2 reduction4–8 due to its powerful utilization of solar energy.It is well known that the photocatalytic efficiency of semi-conductors is greatly affected by either the photocatalytic sensitivity in the absorbing light region or the rate recombination of photogenerated electron–hole pairs.9–11 Visible light occupies 96% of the sun's light, so semiconductors with the band gap in the visible region may be more suitable for practical application.12 Except for hunting new materials with appropriate band gap, it is of great significance to explore the compounds of existing materials for new properties.13–16
Rare earth orthovanadates, which is an important family of inorganic materials, has a great potential in application of various fields, such as catalysts, polarizers, laser host materials and phosphors.17 Cerium orthovanadate, CeVO4, is one of advanced and promising photocatalytic materials due to its chemical stability and non-toxicity.18,19 However, low efficiency of light absorption and rapid recombination with photo-generated electron–hole pairs limit its practical application.20 Therefore, an appropriate composite structure of semiconductor and other substances, such as CNT, graphite-like C3N4 and graphene oxide (GO), may improve photocatalytic activity.21
Graphene oxide, as a novel one-atom-thick graphitic carbon system, has been pursued as a support material due to its unusual high surface area of 2600 m2 g−1, potential low manufacturing cost, excellent thermal and electrical properties.22,23 The incorporation of GO into semiconductor photocatalysts has been proved to be an effective strategy to improve their performance.24,25 The existence of suitable amount of functional groups (such as hydroxyl, epoxy, carbonyl and carboxyl groups) on GO surface ensures that GO could intimately contact with the semiconductor photocatalysts, promoting the separation of the photo-generated charge carriers in semiconductors.26,27
However, GO–semiconductor nanocomposites with powder morphology in aqueous photocatalytic systems are difficult to be separated and recycled from the reaction system. To overcome this obstacle, three-dimensional (3D) graphene macrostructures (hydrogels and aerogels) composed of a unique 3D porous graphene skeleton have been developed recently.28 Photocatalysts with a (3D) porous structure have extremely light weight, which enables them to float on the surface of the reaction system and absorb more solar irradiations. Meanwhile, they can be easily recycled.29
Our recent studies also demonstrated that incorporating CeVO4 into rGO-based aerogel can be an ideal way for developing visible-light photocatalysts.30 However, the method requires complicated multi-step synthesis in our previous study. Herein, we report a one-step hydrothermal method to synthesize CeVO4/rGO composite aerogels in which CeVO4 particles in situ grow on the GO sheets. The as-prepared CeVO4/rGO aerogels show excellent performance in the photocatalytic degradation of methylene blue (MB) under visible light. These aerogels floating on water can be recycled and reused with unvaried performance.
2. Experimental
2.1 Preparation
Graphene oxide (GO) was synthesized using a modified Hummers methods from graphite flake.30 Typically, for preparation of CeVO4/rGO composite aerogel, GO aqueous dispersion (50 ml, 3 mg ml−1) was sonicated for 1 h. After that, 0.00025 mol NH4VO3 was dissolved in 10 ml H2O, and then added into the GO solution to form a mixed solution. Subsequently, 10 ml 0.00025 mol Ce(NO3)3·5H2O was dripped into the above solution under vigorous stirring, and then stirred for 2 h. The pH value of the whole solution was adjusted to 8.8. The resultant solution was transferred into a Teflon-sealed autoclave and heated at 180 °C for 20 h to obtain the hydrogel. Then, the as-prepared hydrogels were washed in deionized water to remove the residual ions. Finally, the hydrogels were freezing dried to form aerogels. Pure CeVO4 powders were prepared similarly in absence of GO.2.2 Characterization
The phase structures of the products were collected on a Bruker D8 Advance X-ray diffractometer (Cu Kα radiation, λ = 0.15406 nm) in a 2θ range from 10° to 60°. The morphologies of the as-synthesized products were examined by field-emission scanning electron microscopy (FESEM, JEOL, JSM-7001F) and transmission electron microscopy (TEM, JEOL, JEM-2100). Raman experiments were performed using a DXR spectrometer using the 532 nm laser line and measurements were made in backscattering geometry. The surface electronic states were analyzed using X-ray photoelectron spectroscopy (XPS, Perkin-Elmer PHI 5000C). N2 adsorption/desorption isotherms were obtained by a Brunauer–Emmett–Teller apparatus (BET, BELSORP-miniII). EIS measurement was carried out on Electrochemiluminescence analyzer (RFL-1) using a three electrode configuration with the as-prepared samples as working electrodes, a Pt foil as counter electrode and a standard calomel electrode as reference electrode. The fluorescence (FL) spectra were surveyed by a Hitachi FL 4600 using the PMT voltage of 700 V to study the recombination efficiency of photo-generated electrons and holes. UV-vis diffuse reflectance spectra (UV-vis DRS) were achieved on a UV-visible spectrophotometer (Shimadzu UV-2450) using BaSO4 as the reference. The absorbency of solution was tested on a Shimadzu UV-2550 spectrophotometer at room temperature.2.3 Photocatalytic test
Photocatalysis was evaluated through the photodegradation of methylene blue (MB) under xenon light (simulated solar light irradiation). In a typical reaction, the CeVO4/rGO aerogel was placed in 10 mg l−1 of MB solution, then the suspension was stirred for 30 min in the dark to establish an adsorption–desorption equilibrium. Since the CeVO4/rGO aerogel floated on the surface of water, the photocatalytic reaction was carried out without stirring. A 4 ml solution was taken at 3 min intervals during the experiment. The resulting solution was analyzed by UV-vis spectrophotometer. The degradation percentage of the dyes is defined as:
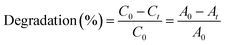 | (1) |
3. Results and discussion
To optimize the reaction condition for synthesizing CeVO4/rGO composite aerogels, a serial concentration from 0.5 to 3 mg ml−1 was tested and the reaction condition was setup at 180 °C for 20 h. Two kinds of products with distinct appearance were obtained, as shown in Fig. 1a. Suspensions of black powdery materials were formed when the GO concentration was at 0.5 and 1 mg ml−1. Because low concentration of GO could reduce the contact opportunity between graphene nanosheets, it is feasible to aggregate and precipitate into powders. Correspondingly, 3D hydrogels were formed after increasing GO concentration to 2 and 3 mg ml−1. Here, under the GO concentration of 3 mg ml−1, the reaction was conducted at different hydrothermal temperature and time (as shown in Fig. 1b and c). The results showed that 3D hydrogels could be formed under all tested temperature and time when the GO concentration kept at 3 mg ml−1, indicating that the high concentration of GO is crucial to form 3D CeVO4/rGO composite hydrogels rather than reaction temperature and time. We proposed the putative mechanism as shown in Fig. 1d. 3D hydrogel can be formed with GO sheets through a self-assembly process under hydrothermal conditions. Meanwhile, CeVO4 particles were also grown and anchored on the rGO sheets with the simultaneous reduction of GO. Importantly, high concentration of GO is necessary for 3D hydrogel formation, since which could enhance the cross-linking of GO sheets by the combination of p–p stacking and hydrophobic interactions.31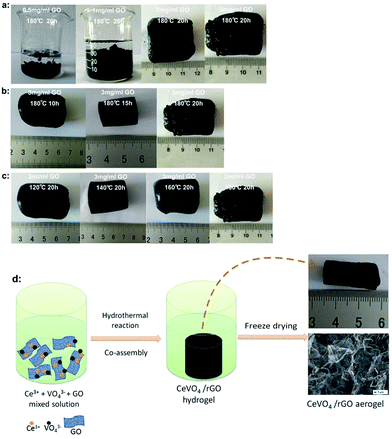 | ||
| Fig. 1 (a–c) The photographs of the products synthesized under different conditions (d) the formation mechanism of CeVO4/rGO composite aerogels. | ||
The phase structure of the products was investigated by XRD. Fig. 2a–c showed XRD patterns of the resulting products synthesized under different reaction conditions. All diffraction peaks at the 2θ values of 18.11, 23.91, 32.43, 34.22, 39.07, 43.54, 46.41, 48.01, 49.32 and 55.64 can be indexed to the (101), (200), (112), (301), (103), (321), (312), (400) and (004) crystal planes of tetragonal CeVO4 (JCPDS 29-0398).32 The diffraction peak of graphite was not observed due to the effective intercalation.33 None of impurities was detected in these patterns. All the diffraction peaks were sharpened as the reaction time or temperature increased, indicating the importance of these reaction factors in improvement of crystalline degree of CeVO4 particles.
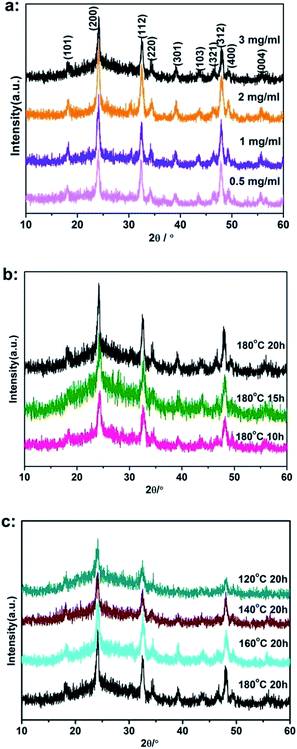 | ||
| Fig. 2 XRD patterns of the CeVO4/rGO composite aerogels prepared under different reaction conditions of (a) GO concentration, (b) reaction time, (c) reaction temperature. | ||
Raman spectroscopy was used to confirm the formation of CeVO4 phase and rGO sheets. The Raman spectra of the bare CeVO4 powders and CeVO4/rGO composite aerogels obtained from reaction with different GO concentration were shown in Fig. 3. For CeVO4, bands at 849 and 772 cm−1 correspond to the symmetric (A1g) and anti-symmetric (B1g) stretching of VO43− tetrahedrons.34 Those at 464 and 375 cm−1 can be specified as B1g and A1g bending modes. The B2g bending mode of VO43− tetrahedrons can be detected at 263 cm−1.35 For CeVO4/rGO aerogels, two additional peaks were observed at 1352 and 1588 cm−1, which are the characteristic signals of disorder band associated with structural defects generated in graphene (D band) and E2g phonon scattering of the well-ordered sp2-bonded carbon atoms of graphene (G band), respectively.36 All of the characteristics from Raman spectra indicated that CeVO4 particles can in situ grow on the surface of rGO sheets in the CeVO4/rGO composite aerogels.
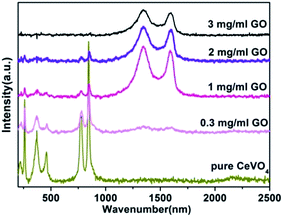 | ||
| Fig. 3 Raman spectra of bare CeVO4 and CeVO4/rGO composite aerogels obtained with different GO concentration (180 °C, 20 h). | ||
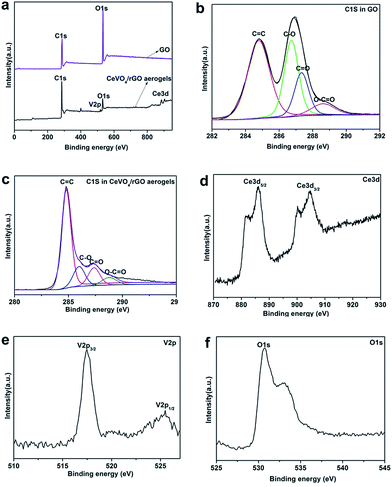
To investigate the surface nature, XPS was carried out to detect the surface components of the GO sheets and the CeVO4/rGO aerogel. The survey of XPS spectrum for the GO sheets indicated that elemental C and O occurred, evidencing that the graphite was oxidized. Meanwhile, Ce 3d, V 2p, O 1s and C 1s could be detected in the CeVO4/rGO aerogel (Fig. 4a). High resolution C 1s peaks of GO can be deconvoluted into sp2-hybridized carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), epoxy/hydroxyl carbon (C–O), carbonyl (C
C), epoxy/hydroxyl carbon (C–O), carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and carboxyl (O–C
O) and carboxyl (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bonds at binding energies of 284.8, 286.7, 287.4 and 288.6 eV, respectively37 (Fig. 4b).
O) bonds at binding energies of 284.8, 286.7, 287.4 and 288.6 eV, respectively37 (Fig. 4b).
While for the CeVO4/rGO aerogel, the C 1s spectrum showed a major peak at 284.8 eV, and peaks corresponding to the C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds were significantly weakened, suggesting effective reduction of the GO sheets (Fig. 4c). Meanwhile, the Ce 3d spectrum of the composite was also presented in Fig. 4d. The main peaks at 881.4 and 886.1 eV were assigned to the Ce 3d5/2 binding energy, whereas the peaks centred at 900.2 and 904.1 eV were ascribed to the Ce 3d3/2 binding energy.38 The V 2p spectrum demonstrated that the two characteristic states, V 2p3/2 and V 2p1/2, were assigned to the dominant pentavalent state V5+ (Fig. 4e).38 The photoelectrons from O 1s displayed a relatively wide, asymmetric peak, with the highest point at 530.8 eV, which could be assigned to the oxygen bond with V atoms in CeVO4 (Fig. 4f). The other peak at lower photoelectron energy of 533.2 eV may be attributed to the C–O bond originating from the residual oxygen-containing groups.39
O bonds were significantly weakened, suggesting effective reduction of the GO sheets (Fig. 4c). Meanwhile, the Ce 3d spectrum of the composite was also presented in Fig. 4d. The main peaks at 881.4 and 886.1 eV were assigned to the Ce 3d5/2 binding energy, whereas the peaks centred at 900.2 and 904.1 eV were ascribed to the Ce 3d3/2 binding energy.38 The V 2p spectrum demonstrated that the two characteristic states, V 2p3/2 and V 2p1/2, were assigned to the dominant pentavalent state V5+ (Fig. 4e).38 The photoelectrons from O 1s displayed a relatively wide, asymmetric peak, with the highest point at 530.8 eV, which could be assigned to the oxygen bond with V atoms in CeVO4 (Fig. 4f). The other peak at lower photoelectron energy of 533.2 eV may be attributed to the C–O bond originating from the residual oxygen-containing groups.39
FE-SEM images of cross-section of CeVO4/rGO aerogel exhibited that this 3D composite has an interconnected hierarchical porous network structure with pore sizes in the range of submicrometers to ten micrometers (Fig. 5a and b). Moreover, a large number of CeVO4 particles with rectangular shape anchored on graphene sheets and distributed in an even and dense way (Fig. 5c), and sizes of which ranged from 20 to 40 nm. High-resolution TEM image (Fig. 5d) of a single rectangular CeVO4 particle showed that the plate was well-crystallized with the (200) fringes running along the plate direction and spaced by 0.37 nm. The density of the CeVO4/rGO aerogel was calculated to be 0.11 g cm−3, which was comparable to the literature value (0.16 g cm−3).40
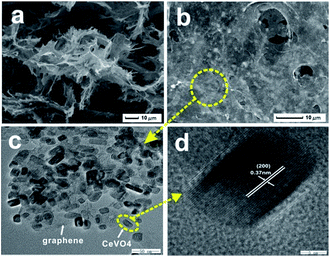 | ||
| Fig. 5 (a and b) SEM and (c) TEM images of CeVO4/rGO composite aerogel (180 °C, 20 h, 3 mg ml−1 of GO), (d) high resolution TEM image of CeVO4 particles in the aerogel. | ||
The surface area and porosity characteristics of pure CeVO4 and CeVO4/rGO aerogel were obtained by N2 adsorption–desorption experiments. The specific surface area was measured using the BET method, and the BET specific surface areas of CeVO4 and CeVO4/rGO aerogel were calculated to be 5.9 and 143.2 m2 g−1, respectively. The difference of the surface area was mostly ascribed to the poriferous structure of aerogel. The increased specific surface areas can absorb more reactants on the surface of catalyst, which could improve the photocatalytic performance. The adsorption–desorption curves for CeVO4 and CeVO4/rGO were shown in Fig. 6a, it can be seen that the CeVO4/rGO aerogel showed a typical isotherm curve of type-IV behaviour and the definite hysteresis corresponding to the existence of mesopores. The pore size distribution was calculated using the classical Barrett–Joyner–Halenda (BJH) model (Fig. 6b), and the mesopore diameters of the CeVO4/rGO arising from the intervoids of rGO sheets were estimated as 37.94 nm. Therefore, the larger surface area and the 3D macroporosity of the graphene aerogel greatly improved the performance in photocatalysis.
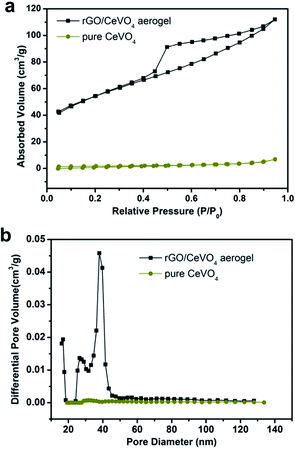 | ||
| Fig. 6 (a) N2 adsorption/desorption isotherms and (b) pore size distribution curves of CeVO4 and CeVO4/rGO aerogels. | ||
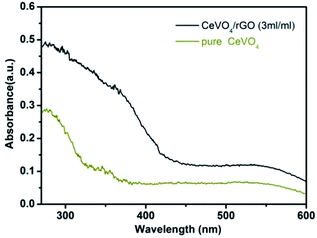
The UV-visible absorbance spectra of the as-synthesized pure CeVO4 and CeVO4/rGO aerogel were presented in Fig. 7. The spectrum of the pure CeVO4 showed an absorption edge corresponding to a bandgap of 3.47 eV. Compared with pure CeVO4, the introduction of rGO not only enhanced absorption in the visible light region, but also shifted absorption maxima to the higher wavelength region (red shift), which may be due to the absorption contribution from the graphene sheets and modification of the fundamental process of exciton formation upon irradiation.41 These phenomena also suggested strong coupling between the graphene sheets and CeVO4.
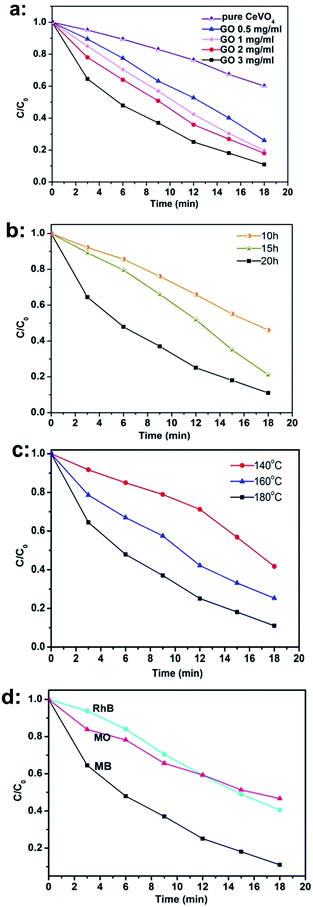
Before the photocatalytic test, the adsorption property of the aerogels was characterized and the results were shown in Fig. S1 (see ESI†). It can be seen that all of the CeVO4/rGO aerogels showed higher adsorption ability than that of pure CeVO4. To investigate the photocatalytic efficiency of CeVO4/rGO aerogels, their degradation on MB was performed under visible light irradiation (Fig. 8a). Compared with bare CeVO4 powders, the photocatalytic degradation efficiency of CeVO4/rGO aerogels was dramatically increased, indicating that the photocatalytic efficiency of CeVO4 could be enhanced through incorporation of GO. The superior activity of aerogels over the bare CeVO4 powders was mainly accredited to two reasons.
Firstly, the aerogels have larger surface area and higher pore volume. Large surface area and high pore volume were benefit for adsorbing enough active sites and reactant, and thus to promote photocatalytic reactions.42 Secondly, the surface functional groups (especial carboxyl and hydroxyl group) on GO sheets were able to promote photo-induced electron transfer between active sites and carbon atoms, and then the photocatalytic activity was increased. The catalytic performances of the CeVO4/rGO aerogels synthesized under different reaction condition were also examined, showing that the photocatalytic performance could be enhanced by increase of GO concentration. As reported, the surface functional groups on GO sheets can enhance the interfacial interaction, and higher GO concentration offered more chances for separating the photo-generated electron–hole pairs.31 Besides that, an increase of the degradation efficiency was also observed along with the increase of reaction temperature and time (Fig. 8b and c), and this enhanced performance may be ascribed to the improved combination between CeVO4 particles and GO sheets. As noted, CeVO4/rGO aerogels (180 °C, 20 h, 3 mg ml−1 of GO) displayed the best photocatalytic performance. The CeVO4/rGO aerogel (180 °C, 20 h, 3 mg ml−1 of GO) was also utilized to degrade Rhodamine B (RhB) and methyl orange (MO) (Fig. 8d). Obviously, the CeVO4/rGO aerogel exhibited different activities of photo-degradation on different pollutants. For example, the degradation rate was 59.5% for RhB within 18 min while 53.5% for MO. The as-prepared CeVO4/rGO aerogel possessed a unique hollow macroporous structure with large surface area, which can provide more active sites for the adsorption and reaction of dyes, whereas the hierarchical architecture was able to more effectively transport reactant and product molecules. Besides that, this structure also favoured multilight scattering/reflection, resulting in enhanced harvesting of exciting light.43–45 As a result, the CeVO4/rGO aerogel demonstrated greatly improved photocatalytic activity.
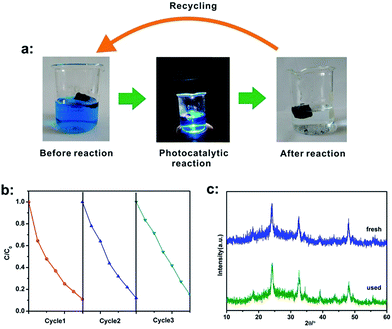
Since stability and reusability of catalysts are very important issues for practical applications, the recyclability of CeVO4/rGO aerogel (180 °C, 20 h, 3 mg ml−1 of GO) for the catalytic reduction of MB was tested. This recycling process was shown in Fig. 9a. After the reaction, the aerogel was removed and washed with filter paper, and then it could be reused for further catalytic reactions. As shown in Fig. 9b, the activity of the aerogel did not significantly attenuate even after three photodegradation cycles of MB. Furthermore, XRD patterns of the fresh and used CeVO4/rGO aerogel were shown in Fig. 9c. There was no obvious difference between the used and fresh aerogels, and no other phase was observed after the photocatalytic activity, indicating that the CeVO4/rGO aerogel was stable and reusable.
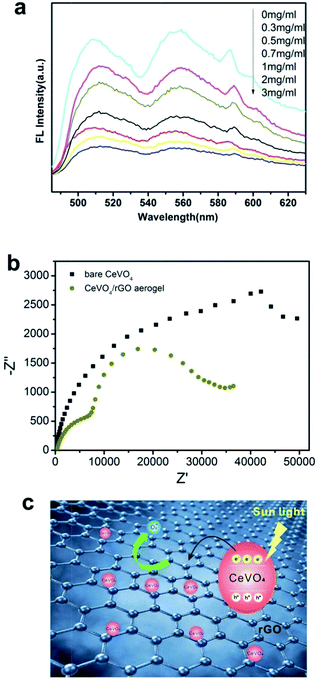
In order to study the possibility of recombination for photo-generated electron–hole pairs, the fluorescence (FL) spectra measurement of bare CeVO4 and CeVO4/rGO aerogels synthesized using different GO concentrations was carried out. The results showed that bare CeVO4 had the highest fluorescence (FL) intensity compared with the FL intensity of the as-prepared CeVO4/rGO aerogels (Fig. 10a). The FL intensity was suppressed when GO sheets assembled into a 3D frame, demonstrating that this special graphene-based structure can promote electron separation and transfer effects. The FL intensity of CeVO4/rGO aerogels was also observed to be decreased with the increasing concentration of GO. In particular, CeVO4/rGO aerogel synthesized with 3 mg ml−1 GO had the lowest FL intensity. As FL emission mainly results from the recombination of excited electron–hole pairs, a lower FL intensity indicates a lower recombination rate of electron–hole pairs under the same test conditions.46–48 The results suggested that the CeVO4/rGO aerogel (3 mg ml−1) showed the minimal efficiency of recombination of photo-generated electron–hole pairs, contributing to its highest photocatalysis. Fig. 10b displayed the Nyquist plots of electrochemical impedance spectroscopy (EIS). In Nyquist plots, the pure CeVO4 showed the smaller semicircle in the middle-frequency region compared with that of CeVO4/rGO aerogel (3 mg ml−1 GO) under light irradiation, indicating that the introduction of rGO sheets promoted the electrons migration. A proposed mechanism for the photocatalysis of CeVO4/rGO aerogels under natural sunlight was shown schematically in Fig. 10c. Under sunlight irradiation, the VB electrons of CeVO4 were excited to the CB, generating holes in the VB. The rGO sheets in the CeVO4/rGO aerogels could act as electron acceptors, leading to an effective separation of the photo-generated electron–hole pairs. Graphene had high photogenerated electrons mobility at room temperature, so the electrons can mobilize on the graphene sheet, resulting in the inhibition of the photogenerated electron–hole pair recombination by collecting the electrons.32 The electron–hole separation and transportation could occur at the interface of CeVO4/rGO aerogels. Along with the separation of electrons and holes, these electrons could further contact with O2 to produce superoxide (O2−) radicals, while most of these holes as oxidizers could directly react with dye molecules.
4. Conclusion
In summary, 3D porous CeVO4/rGO aerogels were prepared, and their photocatalytic activities were evaluated. It was found that CeVO4 particles with a diameter of 20–40 nm could in situ grow well on the rGO sheets with close interfacial contacts, and the rGO sheets then self-assemble into a 3D dimensional framework. The as-prepared CeVO4/rGO aerogels exhibited higher photocatalytic activity than that of bare CeVO4 particles. This is mainly attributable to the special structure of the aerogel that composed of graphene sheets with the larger surface area and hierarchical porosity, by which transport of reactant and product molecules is increased, harvesting of exciting light is enhanced and the electron–hole recombination is reduced. Moreover, CeVO4/rGO aerogels showed great stability in photocatalytic activity, and they can float on water and be easily recycled with high efficiency. Therefore, this simple hydrothermal technique for synthesizing recyclable semiconductor–graphene aerogels will be utilized promisingly and potentially in water treatment.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51202093, 51302111 and 51272093), Science Foundation of Jiangsu Province (No. BK20130523), Jiangsu University Development Foundation for Talents (No. 11JDG025), Jiangsu University Postdoctoral Science Foundation (No. 1143002079), Jiangsu Province Postdoctoral Science Foundation (No. 1101035C), China Postdoctoral Science Foundation (No. 20110491356).References
- B. C. Qiu, M. Y. Xing and J. L. Zhang, J. Mater. Chem. A, 2015, 3, 12820–12827 CAS.
- Q. Q. Liu, C. Y. Fan, H. Tang, X. J. Sun, J. Yang and X. N. Cheng, Appl. Surf. Sci., 2015, 358, 188–195 CrossRef CAS.
- H. Tang, H. Huang, X. S. Wang, K. Q. Wu, G. G. Tang and C. S. Li, Appl. Surf. Sci., 2016, 379, 296–303 CrossRef CAS.
- S. Wang and X. Wang, Angew. Chem., Int. Ed., 2016, 55, 2308–2320 CrossRef CAS PubMed.
- S. Wang, W. Yao, J. Lin, Z. Ding and X. Wang, Angew. Chem., Int. Ed., 2014, 53, 1034–1038 CrossRef CAS PubMed.
- S. Wang and X. Wang, Small, 2015, 11, 3097–3112 CrossRef CAS PubMed.
- S. Wang, Z. Ding and X. Wang, Chem. Commun., 2015, 51, 1517–1519 RSC.
- S. Wang, Y. Hou and X. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 4327–4335 CAS.
- D. Wu, S. T. Yue, W. Wang, T. C. An, G. Y. Li, H. Y. Yip, H. J. Zhao and P. K. Wong, Appl. Catal., B, 2016, 192, 35–45 CrossRef CAS.
- W. C. Wan, S. Yu, F. Dong, Q. Zhang and Y. Zhou, J. Mater. Chem. A, 2016, 4, 7823–7829 CAS.
- X. Xu, F. W. Ming, J. Q. Hong, Y. Q. Xie and Z. C. Wang, Mater. Lett., 2016, 179, 52–56 CrossRef CAS.
- X. B. Li, S. W. Yang, J. Sun, P. He, X. G. Xu and G. Q. Ding, Carbon, 2014, 78, 38–48 CrossRef CAS.
- Q. Li, X. Li, S. Wageh, A. A. Al-Ghamdi and J. G. Yu, Adv. Energy Mater., 2015, 5, 1500010 CrossRef.
- W. Zhang, F. Dong and W. Zhang, Appl. Surf. Sci., 2015, 358, 75–83 CrossRef CAS.
- X. Li, J. Yu, J. Low, Y. Fang, J. Xiao and X. Chen, J. Mater. Chem. A, 2015, 3, 2485–2534 CAS.
- T. Xiong, F. Dong, Y. Zhou, M. Fu and W. Ho, J. Colloid Interface Sci., 2015, 447, 16–24 CrossRef CAS PubMed.
- Y. Li, Z. H. Sun, S. M. Zhu, Y. L. Liao, Z. X. Chen and D. Zhang, Carbon, 2015, 94, 599–606 CrossRef CAS.
- Y. Q. Shen, Y. C. Huang, S. J. Zheng, X. F. Guo, Z. X. Chen, L. M. Peng and W. P. Ding, Inorg. Chem., 2011, 50, 6189–6194 CrossRef CAS PubMed.
- X. J. Yang, W. L. Zuo, F. Li and T. H. Li, ChemistryOpen, 2015, 4, 288–294 CrossRef CAS PubMed.
- N. Ekthammathat, T. Thongtem, A. Phuruangrat and S. Thongtem, J. Nanomater., 2013, 434, 197–204 Search PubMed.
- J. Wei, S. L. Xue, P. Xie and R. J. Zou, Appl. Surf. Sci., 2016, 376, 172–179 CrossRef CAS.
- Y. Ding, Y. F. Zhou, W. Y. Nie and P. P. Chen, Appl. Surf. Sci., 2015, 357, 1606–1612 CrossRef CAS.
- R. O. Silva, F. J. Heiligtag, M. Karnahl, H. Junge, M. Niederberger and S. Wohlrab, Catal. Today, 2015, 246, 101–107 CrossRef.
- S. P. Dubey, A. D. Dwivedi, I. C. Kim, M. Sillanpaa, Y. N. Kwon and C. Lee, Chem. Eng. J., 2014, 244, 160–167 CrossRef CAS.
- W. J. Liu, J. Y. Cai and Z. H. Li, ACS Sustainable Chem. Eng., 2015, 3, 277–282 CrossRef CAS.
- M. M. Wang, G. L. Cao and M. Shao, Phys. Chem. Chem. Phys., 2015, 17, 24901–24907 RSC.
- R. D. Amaranatha, C. Jiha and L. Seunghee, RSC Adv., 2015, 5, 67394–67404 RSC.
- Y. Hou, Z. H. Wen, S. M. Cui, X. L. Feng and J. H. Chen, Nano Lett., 2016, 16, 2268–2277 CrossRef CAS PubMed.
- J. Y. Cai, W. J. Liu and Z. H. Li, Appl. Surf. Sci., 2015, 358, 146–151 CrossRef CAS.
- C. Y. Fan, Q. Q. Liu, T. D. Ma, J. Y. Shen, Y. Yang, H. Tang, Y. P. Wang and J. Yang, Ceram. Int., 2016, 42, 10487–10492 CrossRef CAS.
- Y. Y. Fan, W. G. Ma, D. X. Han, S. Y. Gan, X. D. Dong and N. Li, Adv. Mater., 2015, 27, 3767–3773 CrossRef CAS PubMed.
- R. K. Selvan, A. Gedanken, P. Anilkumar, Æ. G. Manikandan and C. Karunakaran, J. Cluster Sci., 2009, 20, 291–305 CrossRef.
- J. Ma, C. Yang, C. Na and F. Yu, New J. Chem., 2016, 40, 3208–3215 RSC.
- A. Phuruangrat, B. Kuntalue, S. Thongtem and T. Thongtem, Mater. Lett., 2016, 174, 138–141 CrossRef CAS.
- F. Luo, C. J. Jia, R. Liu, L. D. Sun and C. H. Yan, Mater. Res. Bull., 2013, 48, 1122–1127 CrossRef CAS.
- T. Wu, M. X. Chen, L. Zhang, X. Y. Xu, Y. Liu, J. Yan, W. Wang and J. P. Gao, J. Mater. Chem. A, 2015, 3, 12820–12827 Search PubMed.
- J. M. Hou, H. H. Huang, Z. Z. Hanb and H. B. Pan, RSC Adv., 2016, 6, 14552–14558 RSC.
- X. Xu, H. Li, Q. Q. Zhang, H. Hu, Z. B. Zhao, J. H. Li, J. Y. Li and Y. Qiao, ACS Nano, 2015, 9, 3969–3977 CrossRef CAS PubMed.
- X. Li, S. Yang, J. Sun, P. He, X. Xu and G. Ding, Carbon, 2014, 78, 38–48 CrossRef CAS.
- H. Sun, Z. Xu and C. Gao, Adv. Mater., 2013, 25, 2554–2560 CrossRef CAS PubMed.
- D. Reddy, J. Choi, S. Lee, R. Ma and T. K. Kim, RSC Adv., 2015, 5, 18342–18351 RSC.
- V. H. Luan, H. N. Tien and S. H. Hur, J. Colloid Interface Sci., 2015, 437, 181–186 CrossRef CAS PubMed.
- T. Zhao, Z. Liu, K. Nakata, S. Nishimoto, T. Murakami, Y. Zhao, L. Jiang and A. Fujishima, J. Mater. Chem., 2010, 20, 5095–5099 RSC.
- B. Fang, Y. Xing, A. Bonakdarpour, S. Zhang and D. P. Wilkinson, ACS Sustainable Chem. Eng., 2015, 3, 2381–2388 CrossRef CAS.
- B. Fang, A. Bonakdarpour, K. Reilly, Y. Xing, F. Taghipour and D. P. Wilkinson, ACS Appl. Mater. Interfaces, 2014, 6, 15488–15498 CAS.
- J. M. Hou, H. H. Huang, Z. Z. Han and H. B. Pan, RSC Adv., 2016, 6, 14552–14558 RSC.
- S. Wang and X. Wang, Appl. Catal., B, 2015, 162, 494–14558 CrossRef CAS.
- S. Wang, J. Lin and X. Wang, Phys. Chem. Chem. Phys., 2014, 16, 14656–14660 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra18013b |
| This journal is © The Royal Society of Chemistry 2016 |