High performance ternary solar cells based on P3HT:PCBM and ZnPc-hybrids
Abstract
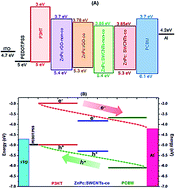
Single walled carbon nanotubes (SWCNTs) and reduced graphene oxide (rGO) covalently and non-covalently functionalised by zinc phthalocyanine (ZnPc) were added to P3HT:PCBM blend in order to investigate the effects of these hybrid materials on P3HT:PCBM organic solar cell performance. Adding a small amount of these hybrids to P3HT:PCBM blend does not significantly alter the absorption spectra of the latter nor its structure. ZnPc–rGO and ZnPc–SWCNT hybrid features have appeared on the P3HT:PCBM surface morphology as verified by SEM and AFM images. However these hybrid materials have caused significant effects on the electrical properties of the studied blends. An increase of about two orders of magnitudes has been observed in the electrical conductivity. Space charge limited conduction theory was employed to investigate the charge carriers' mobility whereas a thermionic emission model was used to evaluate the recombination rate based on an estimated diode ideality factor. Solar cell devices based on P3HT:PCBM:ZnPc–SWCNTs-co bonded have demonstrated best device performance with PCE of 5.3%, Jsc of 12.6 mA cm−2, Voc of 0.62 V and FF of 68%. A reference device based on bare P3HT:PCBM blend has exhibited PCE of just under 3.5%, Jsc of 9.3 mA cm−2, Voc of 0.62 V and FF of 60%.

 Please wait while we load your content...
Please wait while we load your content...