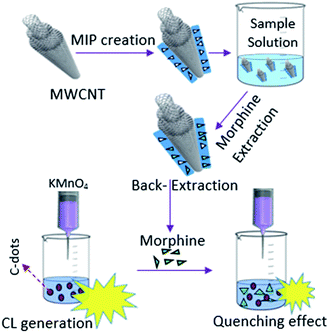
Molecularly imprinted polymers on multi-walled carbon nanotubes as an efficient absorbent for preconcentration of morphine and its chemiluminometric determination†
Ali Lotfia,
Sepideh Karimib and
Javad Hassanzadeh*a
aYoung Researchers and Elite Club, Tabriz Branch, Islamic Azad University, Tabriz, Iran. E-mail: javadhassanzadeh63@gmail.com; Fax: +98 4133333458; Tel: +98 9146127692
bDepartment of Chemistry, Varamin (Pishva) Branch, Islamic Azad University, Varamin, Iran
First published on 26th September 2016
Abstract
A simple and selective method was described for the rapid determination of morphine based on its extraction and preconcentration by molecularly imprinted polymers (MIPs) on multi-walled carbon nanotubes (MWCNTs) prior to its chemiluminometric recognition. MIP–MWCNTs composite was obtained by copolymerization of certain monomers on the surface of vinyl modified MWCNTs using morphine as template molecule. Dispersed MIP–MWCNTs indicated good tendency and selectivity to low amounts of morphine in relatively acidic condition. Some important factors containing buffer and its pH, ionic buffer, incubation time, eluent, etc., were optimized for extraction step. KMnO4–carbon dots (C-dots) chemiluminescence (CL) reaction was applied as recognition device for extracted morphine. Morphine can linearly quench the CL emission of this system via its interaction with C-dots. The calibration graph was obtained in the range of 0.003–1.2 mg L−1 morphine concentration with a detection limit (3 s) of 0.82 μg L−1. The relative standard deviation (RSD%) for five determinations of 0.01, 0.1, and 0.8 mg L−1 morphine were 1.11%, 2.54% and 1.98%, respectively. The developed method has acceptable features in comparison with other reported methods and was satisfactorily applied to the accurate determination of morphine in pharmaceutical and urine samples.
1. Introduction
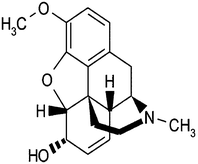
Morphine (5a,6a-didehydro-4,5-epoxy-17-methylmorphinan-3,6-diol) (Fig. 1), as a potent opioid analgesic drug, is most frequently applied to the effective and short-term control of post-surgery pain in clinical medicine.1–3 World Health Organization (WHO) also recommended it for the relief of moderate cancer-related pains.4 Morphine is an important opiate and abused as an illicit drug, which can be obtained from some herbal sources such as opium, poppy straw concentrate, and other poppy derivatives. However, when it is abused in excess amounts, toxication symptoms and disruption effect in the central nervous system can be observed. Very high doses of morphine (120 mg) can lead to death.4 Sensitive monitoring methods are necessary for morphine detection in human biological samples to prevent overdose-induced toxication and its abuse.4,5 Moreover, morphine analysis can be useful as an interesting topic in clinical and forensic fields to study addiction history.4–6 It has been demonstrated that around the 90% of orally consumed morphine is extruded from body within 24 h via urine and only 10% of this morphine is un-metabolized. So, the concentration of morphine in a consumer urine sample would be less than 1 mg L−1.7Various analytical methods have been reported for the determination of morphine, which are often based on gas chromatography (GC),6,8,9 high-performance liquid chromatography (HPLC),5,10–12 with various detection methods, capillary electrophoresis,13,14 chemiluminescence methods,7,15–17 electrochemistry,3,4,18,19 near-infrared spectroscopy,20 immunoassay,2,15 electrochemiluminescence methods,2,21 spectrophotometry,22–24 ion mobility spectrometry,25 thin layer chromatography,26 and surface plasma resonance (SPR).27 Compared with other methods, chemiluminescence techniques were rarely used for the determination of morphine. However, it can offer notable features such as simplicity, versatility, simplified optical setup, rapidity, portability, high sensitivity and low cost. Moreover, several CL methods based on the improving effect of nanoparticles were proposed as an interesting research field in analytical chemistry.28,29 Carbon dots are more attractive new set of nanoparticles due to their lower toxicity, greater photoactivity, and low cost, which are intensely studied in recent times.30
On the other hand, preconcentration techniques can improve the selectivity and facilitate the analysis of complex samples like biological fluids by reducing the influence of interferences. Several methods have been established for the extraction and preconcentration of morphine, for example surfactant enhanced liquid phase microextraction (LPME),31 solid phase microextraction (SPME),32 and solid phase extraction (SPE).33,34 Whereas, SPE is a relatively simple, fast, economic and reliable method for the sample preparation.35–38 The unique properties of SPE have made it a more popular technique to extract trace quantities of various organic and inorganic species from complex media. The main advantages of SPE are high enrichment factor, good recovery, use of small quantities of organic solvents and the possibility of automation of the whole process.35,39,40 Dispersive solid phase extraction (DSPE) has demonstrated as an attractive method which can separate the analyte from the large sample solution volumes.41,42 This is because of the easy and rapid separation of the sorbent (by centrifugation steps) and also the prevention of difficulties such as column packing.41
New variety of materials which can be exploited as solid sorbents has been recently attracted by many researchers. Carbon nanotubes (CNTs) were greatly considered owing to their outstanding mechanical, electrical, and surface features. They have been applied in a wide range of analytical fields, especially for the extraction.43,44 Numerous comparative investigation on the use of MW-CNTs, SWCNTs, C18, activated carbon and silica derivatives as SPE sorbents for the analysis of different analytes have indicated the higher adsorption capacity of nanotubes with better RSD and recovery values.40,41,43,45 The high extraction efficiency of CNTs is related to their high surface/volume ratio. Also, the sorption–elution process is better and faster for CNTs sorbents. Additional notable benefit is the high stability of CNTs that makes it possible to use them under harsh chemical and thermal conditions.40,41,43,45 Furthermore, combining the CNTs with certain recognition element (such as antibodies, enzymes, or aptamers) is an effective way to improve their selectivity and application. Most of the applied recognition systems have some problems in application, such as their incompatibility against organic solvents, high temperatures and pH. In contrast, molecular imprinting strategy, as a biomimetic receptor structure with intended selectivity for target molecule, has outstanding advantages;24,37,38,46–49 simple and rapid preparation, inexpensive, good stability and specificity. The molecularly imprinted polymers (MIPs) as a thin layer on the large surface area of CNTs can adsorb and identify specific and predicted target. The external thin MIPs coating can improve the availability for template molecules to attach the binding sites.
In the current paper, a rapid MIP–MWCNTs based dispersed solid phase extraction method followed by simple chemiluminescence technique was developed for the extraction and determination of ultra-trace quantities morphine in pharmaceutical and human urine samples. MIP–MWCNTs composite was prepared by using copolymerization of methacrylic acid (MAA), ethylene glycol dimethacrylate (EGDMA) and 2,2′-azoisobutyronitrile (AIBN) on the vinyl modified MWCNTs in the presence of morphine as template molecule. The functionalized CNTs can interact with morphine and adsorb it. The high affinity to morphine can lead to its higher preconcentration. The recognition CL system consists of KMnO4–carbon dots reaction which leads to an intensive CL emission.
2. Experimental
2.1. Materials and apparatus
Multi-walled carbon nanotubes (with a diameter of 20–30 nm and a length of 5–15 μm), 2,2-azoisobutyronitrile (AIBN) and ethylene dimethacrylate (EGDMA) were purchased from Aldrich. Other carbon structures including carbon glassy (spherical powder, 2–12 μm), single-walled carbon nanotube (diameter = 1.2–1.7 nm and length = 0.3–5 μm), activated carbon (spherical powder, 10–40 μm) and carbon nanofibers (D × L = 100 nm × 20–200 μm) were also from Aldrich. Morphine hydrochloride was of analytical grade and was obtained from Daru-Pakhsh Co. (Tehran, Iran) and its stock solution (100 mg L−1) was prepared daily by dissolving suitable amounts of morphine hydrochloride powder in deionized water. Phosphate buffer was prepared by dissolving 1.68 mL phosphoric acid (Merck) in deionized water and adjusting its pH using NaOH (Merck) solution (2 mol L−1). Sulfuric acid, hydrochloric acid and other used chemicals were of analytical grade from Merck.Ultraviolet-visible (UV-Vis) spectra and absorptions were recorded on a Lambda 25 Spectrophotometer (Perkin Elmer). The size and structure of the CNTs were confirmed through scanning electron microscopy (SEM, EM 3200, KYKY, China, operated at 26 kV). X-ray diffraction (XRD) analysis was performed with a Philips X-ray powder diffractometer (PW1800) with a Cu Kα radiation (λ = 1.54056 Å) at room temperature. Fourier transform infrared (FTIR) spectroscopy (WQF-510A, Japan) was used to study chemical structure of CNTs.
2.2. Preparation of carbon dots
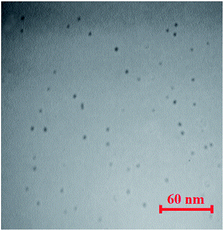
High fluorescent carbon dots (C-dots) were prepared by previously reported method.50 A mixture containing of glycerine (15 mL), poly (ethylene glycol) 1000 (1.0 g, as a passivation agent) and citric acid monohydrate (1.0 g, as a carbon source) was heated in oil bath for 30 min (170 °C). Then, it was cooled and precipitated by acetone. The C-dots was centrifuged and dialyzed against water (dialysis membrane with molecular weight cut-off = 2000 Da) for 2 days to remove unreacted molecules. The product has high mass yield with a concentration of 0.57 mol L−1 in final 50 mL solution. Also, the average size of C-dots was achieved by TEM images (Fig. 2) which was about 5.4 ± 2.3 nm.2.3. Pretreatment of carbon nanotubes
Modification of CNTs was performed according the previously reported work.47 Firstly, 0.1 g multi-walled carbon nanotube was washed by 0.1 mol L−1 NaOH and 0.1 mol L−1 HCl, respectively, to remove all contaminations such as alumina and other metal oxides that are applied as catalyst in carbon nanotube synthesis. Then, to generate carboxylic acid functional groups (–COOH) on the nanotubes, a solution containing equal components of acid sulfuric (1 N) and potassium permanganate (1 N) solution were used for 6 hours in 25 °C. The extra times for oxidation process can ruin or change the nanotube structures.In next step, carboxylic functionalized MWCNTs were dispersed in a mixture containing 10 mL SOCl2 and 30 mL chloroform for 24 h at 60 °C. Then, MWCNTs were washed by THF to remove excess SOCl2. Dried MWNTs–COCl (0.2 g) was then added to 30 mL THF containing 1.16 g allyl alcohol, 0.244 g 4-DMAP (4-dimethylamine pyridine) and 6.06 g triethylamine. It was stirred for 24 h at 50 °C, centrifuged and washed with THF. The obtained solid is vinyl modified MWCNTs which was applied to construct MIP–MWCNTs.
2.4. Synthesis of MIP–MWCNTs
MIP–MWCNTs were formed by selective polymerizing reaction with vinyl groups on the surface of MWCNTs in the presence of morphine as the template molecule. 250 mg of vinyl-MWCNTs was sonicated in 40 mL acetonitrile/methanol (60/40 v/v) solution. Certain amounts of MAA (0.5 mmol), EGDMA (1.5 mmol), AIBN (20 mg) and morphine (0.1 mmol, as template molecule) were added to vinyl-MWCNTs dispersion. The mixture was deoxygenated and then, was sealed and stirred at 60 °C for 18 h to completion of polymerization on the surface of CNTs. The obtained MIPs–MWCNTs was centrifuged and the unreacted procurers were removed by using acetonitrile/methanol mixture. Finally, the trapped morphine molecules were detached from MIPs–MWCNTs by methanol/acetic acid mixture (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The final product was efficiently dried under vacuum and used for extraction aims. A non-imprinted polymer (NIP) was also synthesized using the same method but in absence of template molecule.
1, v/v). The final product was efficiently dried under vacuum and used for extraction aims. A non-imprinted polymer (NIP) was also synthesized using the same method but in absence of template molecule.
2.5. General procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for several times.
1, v/v) for several times.2.6. Sample preparation
Morphine injection samples (10 mg mL−1, Daru-Pakhsh Co.) were directly applied for analysis after some dilution.Urine samples were taken from patients in Tabriz Razi Hospital, who have one 10 mg morphine injection for each 2 day. The samples were collected 2 h after the last morphine injection and kept at −20 °C. For these urine samples, no excess sample pretreatment is necessary, just a sample filtration and a proper dilution were done.
3. Results and discussion
3.1. Carbon nanotubes characterization
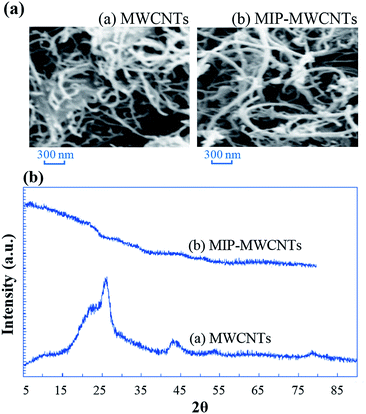
MWCNTs have large surface area and high affinity towards various organic compounds and so, attract more attention as excellent sorbents in solid phase extraction. Fig. 3a show the SEM image for the pretreated MWCNTs.As can be seen, the MWCNTs have a diameter about 30 nm and a length about several micrometers. Polymerization on their surface, a little increase in diameter was observed, which can be due to the MIP generation on MWCNTs (Fig. 3a). Also, X-ray diffraction pattern of MWCNTs–COOH in Fig. 3b, indicates two clear main peaks positioned at 2θ values of about 26° and 42°, well-indexed to the (002), and (100) planes of nanotubes. After MIP creation on the surface of MWCNTs, broad peaks represent the polymer construction (Fig. 3b).
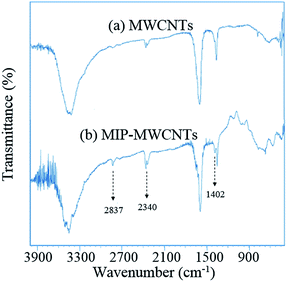
Furthermore, Fourier transform infrared spectroscopy (FTIR) spectra of MWCNTs was showed in Fig. 4. The peaks at 1730 and 2840 cm−1 are related to stretching vibration of carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and C–H bonds, respectively. The hydrogen bond formation between carboxylic groups (–COOH) generate 2 weak peaks at about 2340 and 2320 cm−1. The intense and broad peak appearing at 2900–3600 cm−1 is for (–O–H) bond in carboxylic functional groups. After polymerization experiments, slightly increases can be observed in the intensities of some peaks (Fig. 4); such as 2840 cm−1 related the C–H stretching band, 2340 cm−1 the formed hydrogen bonds and 1402 cm−1 may be related to the C
O) and C–H bonds, respectively. The hydrogen bond formation between carboxylic groups (–COOH) generate 2 weak peaks at about 2340 and 2320 cm−1. The intense and broad peak appearing at 2900–3600 cm−1 is for (–O–H) bond in carboxylic functional groups. After polymerization experiments, slightly increases can be observed in the intensities of some peaks (Fig. 4); such as 2840 cm−1 related the C–H stretching band, 2340 cm−1 the formed hydrogen bonds and 1402 cm−1 may be related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band. These changes indicate the successful coating of molecularly imprinted polymer on the surface of the MWCNTs.
C band. These changes indicate the successful coating of molecularly imprinted polymer on the surface of the MWCNTs.
Here, MIP–MWCNTs with strong adsorption property were chosen for the separation and preconcentration of morphine to achieve the best extraction efficiency. Preliminary experiments showed that MWCNTs have a great capacity for retention and adsorption of morphine (Table 1). This is maybe because of the large surface area of MWCNTs which leads to high efficiency of morphine adsorption. The adsorbed morphine can be eluted by a suitable solvent and then measured using a simple detection method.
| Adsorbent | Enrichment factor |
|---|---|
| Graphene oxide | 79 |
| Glassy carbon | 31 |
| Carbon fibre | 52 |
| Graphene | 83 |
| Activated carbon | 72 |
| SWCNTs | 80 |
| MWCNTs | 100 |
3.2. Chemiluminescence system
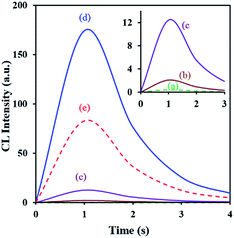
The synthesized C-dots (TEM is shown in Fig. 2) have a UV-vis absorption peak at λ = 300 nm, and can emit an intense fluorescence when excited by an external radiation. The wavelength of observed fluorescence emission was dependent on excitation wavelength and a maximum emission was obtained at 458 nm using an excitation wavelength of 375 nm (Fig. S1†). The quantum yield of synthesized C-dots is 17% achieved by rhodamine B as the reference. The preliminary experiments showed an intense CL emission generation from the direct reaction of C-dots with an oxidant. So, some oxidants containing Ce(IV), K3Fe(CN)6, H2O2, and KMnO4 with various concentrations were tested and the maximum CL intensity was obtained by KMnO4. Also, the effect of some surfactants (Triton X 100, AOT, SDS, and CTAB) was investigated to achieve probable improvement. It is observed that SDS create outstanding promoting effect (Fig. 5). Time profiles for KMnO4–carbon dots–SDS CL system indicate that the maximum CL intensities were achieved within <1 s after injecting KMnO4 (Fig. 5) and so, a very rapid CL reaction was occur between the reagents. Blank experiments by using precursors showed a very low CL intensities.The mechanism for CL reaction was studied by detailing the CL emission spectra (Fig. 6). As can be seen in this figure, a clean peak was attained at about 495 nm which is similar to fluorescence emission spectrum of applied C-dots (Fig. S1, ESI†); it means that the emitting species are probably C-dots. It is capable by recombination of oxidant-injected holes and electrons in carbon dots.30 The red-shift in CL spectra (compared with the fluorescence wavelength) can be described by considering the smaller energy separations of the carbon dots surface states for CL emission.51 Furthermore, CL spectra of KMnO4–carbon dots system contains a second emission peak at 685 nm, which can be related to the Mn(II)* species (from 4T1 to 6A1 transition) produced by reduction of permanganate.51,52
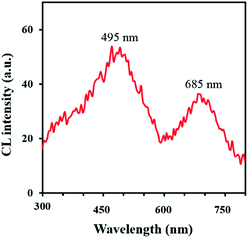 | ||
| Fig. 6 CL spectra of acidic KMnO4–carbon dots–SDS, obtained by continuous flow condition: KMnO4 (1 mmol L−1) in one line and H2SO4 (0.3 mol L−1), SDS (2 mmol L−1) and carbon dots in the other line. | ||
Furthermore, a considerably diminish in the CL intensity of KMnO4–carbon dots–SDS is observed by addition of low quantities of morphine (Fig. 5). It may be due to the attachment of morphine on the surface of C-dots. This claim is due to the observed decrease in the absorption intensity of C-dots in the presence of morphine (Fig. S1a, ESI†). FTIR exhibited the functional groups like methyl, hydroxyl, ether and carboxyl on the surface of C-dots. The attachment of morphine and C-dots is maybe due to their hydrogen bonds or other interactions. The quenching effect of morphine on the CL intensity of KMnO4–carbon dots–SDS was proportional to its concentration and a sensitive method can be designed for the determination of morphine based on this effect. However, the selectivity of CL detection system is not sufficient for this goal. The problem was overcome by using a specific extraction method.
3.3. Optimization
In order to investigate the effect of pH, the extraction of morphine by MIP–MWCNTs was performed in different pH values in the range of 1–10 (Fig. S3a, ESI†). After the extraction of morphine, the absorption of remained solution was recorded. The pH with the lowest absorption intensity was selected as optimum pH, which shows the higher extraction of morphine. As can be seen from the results, pH = 4 provide the best circumstance for morphine extraction. It can be due to the suitable condition of functional groups on the MIP sites of MWCNTs, which leads to a stronger electrostatic interaction with morphine or creation of great hydrogen bonds between them. This results is in good agreement to the previously reported studies.24
The effect of type and concentration of applied buffer to adjust pH = 4 was investigated and the results were shown in Fig. S3b and c (ESI†). The examined buffers include phosphate (Phs), acetate (Act), borate (Bor), tris(hydroxy methyl) amino methane (Tris), and trimethyl ammonium acetate (Tma). It was found that type of buffer significantly affect the extraction results, which is because of changing the ionic strength of solution. Phosphate buffer causes optimum state and was selected for later works.
Also, the effect of the concentration of buffer was studied (Fig. S3c, ESI†). It is clear from the results that 0.005 mol L−1 phosphate buffer cause maximum adsorption of morphine by MIP–MWCNTs.
It is well known that the amount of the absorbent can remarkably affect the extraction efficiency. So, different amounts of MIP–MWCNTs were used as absorbent substrate for the extraction process and it was observed that the use of 0.01 g CNT has led to the maximum elimination of morphine from the working solution (Fig. S3d, ESI†).
Sodium chloride was used as ionic buffer in order to fix ionic strength. It can improve the extraction efficiency by assisting the interactions between the species. Different amounts of NaCl was applied in extraction solution and the results were shown in Fig. S3e (ESI†). It is revealed that 0.2 g NaCl can provide the suitable electrostatic adsorption between morphine and carbon nanotubes. So, the highest adsorption of morphine on the surface of CNTs was obtained.
The extraction time of morphine on the CNTs was also investigated in the range of 2–20 min. The effect of incubation time depends on the reaction rate. Increasing the incubation time improved the extraction efficiency up to about 10 min (Fig. S3f, ESI†). After that the extraction percent was constant. Therefore, 10 min was selected as optimum extraction time for the later extractions.
After adsorption and preconcentration of morphine on the surface of CNTs, in order to determine, the adsorbed morphine should be eluted into a suitable solvent. The type of eluent has significant effect on the morphine determination. It should be able to separate analyte molecules from MIP–MWCNTs as much as possible. So, several solvents were investigated for this purpose. The results were indicated in Fig. S4a (ESI†), which show that ethanol had the best performance and caused more efficient extraction and so high absorption intensities. This is probably owing to the presence of alcoholic functional groups on morphine which can form strong hydrogen bonds with ethanol, leading to effective desorption of morphine molecules from the MIP–MWCNTs. Furthermore, it is found that alkali ethanol improve well the extraction results compared to the acidic ethanol (Fig. 6b), perhaps due to the preventive effect on the protonation of hydroxyl groups on morphine.
For the selected eluent, the effect of its volume was studied. The volume of solvent should be high enough to be able to get more analytes, but, very high amounts of solvent can dilute analyte, resulting to the decreasing the absorption intensity. For this purpose, different volumes of alkali ethanol were applied to separate morphine from MIP–MWCNTs (Fig. S4b, ESI†). The absorption of extracted morphine was enhanced by increasing the eluent volume upto 0.3 mL, then it slightly decreased. So, 0.3 mL alkali ethanol was used as eluent for the experiments.
Finally, the volume of extraction solution was changed to investigate its effect and calculate the limit volume (Fig. S4c, ESI†). Higher volumes than 30 mL of sample solution caused a decrease in absorption of the extracted morphine that it means lower amounts of morphine can be extracted. The reason probably is dilution of morphine and so, it's none efficient interaction with CNTs. Preconcentration factor can be calculated in this step as the ratio of extraction and eluent volumes, which was obtained equal to 100.
By the way, the optimum addition order of reagents are as follows: sample solution, CNTs, NaCl and buffer.
3.4. Analytical application
Based on suitable tendency of prepared MIP–MWCNTs to morphine, a reliable extraction method followed by CL detection was developed for its determination. A schematic image of developed method was shown in Scheme 1. The CL response (ΔI = I0 − I) of the KMnO4–carbon dots–SDS to extracted morphine was plotted in the optimum conditions for its various concentrations (C, mg L−1) as the calibration graph (Fig. 7). The increase in ΔI is proportional to the morphine concentration in the range of 0.003–1.2 mg L−1 (ΔI = 219![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 673C + 57.4, R2 = 0.9998), with detection limit and quantification limit of 0.82 and 2.7 μg L−1, respectively. The relative standard deviations (RSD%) for five determinations of 0.01, 0.1, and 0.8 mg L−1 morphine were 1.11%, 2.54% and 1.98%, respectively.
673C + 57.4, R2 = 0.9998), with detection limit and quantification limit of 0.82 and 2.7 μg L−1, respectively. The relative standard deviations (RSD%) for five determinations of 0.01, 0.1, and 0.8 mg L−1 morphine were 1.11%, 2.54% and 1.98%, respectively.
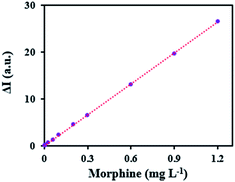 | ||
| Fig. 7 Calibration graph for the determination of morphine by developed method in optimum condition. | ||
Comparison between the applied method and some other reported analytical methods for the morphine quantification is presented in Table S2 (ESI†); the developed extraction system has good linearity and relatively high sensitivity for the determination of morphine. Most of current methods for the determination of morphine are based on chromatographic determinations which have proper sensitivity and precision, but include complicated instrument, and need trained professionals. Moreover, they are time-consuming and expensive.
In addition, the calibration graph for the pure morphine standard solution was obtained by CL system without any preconcentration. The linear range was 0.15–20 mg L−1 (ΔI = 2305C − 79.6, R2 = 0.9962). So, enrichment factor can be calculated for the developed preconcentration as the ratio of two obtained slope, which was equal to about 95.3 times.
3.5. Analysis of real samples
In order to study the applicability of the established method, the process was simply used to the evaluation of morphine in human urine and pharmaceutical samples (Tables 2 and 3). The achieved outcomes have good agreement with a standard technique (high performance liquid chromatography, Tables 2 and 3). Also, to further validate the method, known amounts of morphine standard solution were spiked into the samples prior to any preparation step, and then the samples were evaluated using stated general procedure. Student's t-test results (Tables 2 and 3) indicated that there are no important differences between the added and found values.| Sample | Added (mg L−1) | Founda | Recovery ± RSD | Foundb | tc statis. |
|---|---|---|---|---|---|
| a The mean of five determinations (mg L−1) ± standard deviation (by developed method).b The mean of five determinations (mg L−1) ± standard deviation (by standard method).c t-critical = 2.78 for n = 4 and P = 0.05. | |||||
| Injection | 0 | 9880 ± 2.700 | — | 9911 ± 6.8 | 0.18 |
| 0.5 | 0.491 ± 0.002 | 98.2 ± 2.2 | — | 1.67 | |
| 1 | 0.985 ± 0.012 | 98.5 ± 1.7 | — | 1.81 | |
| 2 | 1.975 ± 0.006 | 98.7 ± 1.3 | — | 2.03 | |
| Sexa/age/illness | Added (mg L−1) | Foundb | Recovery (%) ± RSD | Foundc | td statis. |
|---|---|---|---|---|---|
| a M: male, F: female.b The mean of five determinations (mg L−1) ± standard deviation (by developed method).c The mean of five determinations (mg L−1) ± standard deviation (by standard method).d t-critical = 2.78 for n = 4 and P = 0.05. | |||||
| M/47/addictive | 0 | 6.023 ± 1.687 | — | 6.102 ± 1.240 | 1.73 |
| 13 | 12.777 ± 1.434 | 98.28 ± 1.68 | — | 2.08 | |
| 14 | 14.224 ± 1.787 | 101.60 ± 2.17 | — | 1.45 | |
| 15 | 15.235 ± 2.303 | 101.57 ± 1.22 | — | 2.53 | |
| F/26/addictive | 0 | 7.409 ± 2.059 | — | 7.247 ± 1.581 | 2.38 |
| 13 | 13.221 ± 1.365 | 101.70 ± 1.66 | — | 2.01 | |
| 14 | 14.317 ± 1.909 | 102.26 ± 2.32 | — | 1.91 | |
| 15 | 14.760 ± 1.115 | 98.40 ± 2.12 | — | 1.53 | |
4. Conclusions
A molecular imprinted polymer on the surface of multi-walled carbon nanotubes was generated via selective polymerization of vinyl functional groups. Morphine was applied as template molecule. The produced composite of MIP–MWCNTs was exploited as suitable absorbent to the effective extraction of low quantities of morphine using dispersive solid phase extraction technique followed by elution by alkali ethanol. The offered extraction method showed high selectivity and efficiency. The extracted morphine was determined by using simple and inexpensive chemiluminescence method based on quenching effect of morphine on the CL emission produced by direct reaction of carbon dots and KMnO4. The developed method was applied to the quantification of morphine in pharmaceutical and human urine samples with good agreement to the standard method.Acknowledgements
We would like to express our sincere thanks and gratitude for Young Researchers and Elite Club, Tabriz Branch, Islamic Azad University, Tabriz, Iran, for the financial supports. We would also like to thank the cooperation of the Razi Hospital and Clinical Psychiatry Research Center of Tabriz University of Medical Sciences in the collection and preparation of human urine samples.Notes and references
- P. J. Wiffen, J. E. Edwards, J. Barden and H. J. McQuay, Cochrane Database Syst Rev, 2003, DOI:10.1002/14651858.cd003868, CD003868.
- W. Fei, F. Chen, L. Sun, Q. Li, J. Yang and Y. Wu, Microchim. Acta, 2014, 181, 419–425 CrossRef CAS.
- G. Yang, Y. Chen, L. Li and Y. Yang, Clin. Chim. Acta, 2011, 412, 1544–1549 CrossRef CAS PubMed.
- B. Rezaei, S. Foroughi-Dehnavi and A. A. Ensafi, Ionics, 2015, 21, 2969–2980 CrossRef CAS.
- A. Boojaria, M. Masrournia, H. Ghorbani, A. Ebrahimitalab and M. Miandarhoie, Forensic Sci., Med., Pathol., 2015, 11, 497–503 CrossRef CAS PubMed.
- O. Sabzevari, K. Abdi, M. Amini and A. Shafiee, Anal. Bioanal. Chem., 2004, 379, 120–124 CrossRef CAS PubMed.
- A. M. Idris and A. O. Alnajjar, Talanta, 2008, 77, 522–526 CrossRef CAS.
- M. Barroso, M. Dias, D. N. Vieira, M. López-Rivadulla and J. A. Queiroz, Anal. Bioanal. Chem., 2010, 396, 3059–3069 CrossRef CAS PubMed.
- M. Mátyus, G. Kocsis, O. Boldis, G. Karvaly, E. Magyar, J. Fűrész and A. Gachályi, Acta Chromatogr., 2012, 24, 351–365 CrossRef.
- M. Jafari-Nodoushan, J. Barzin and H. Mobedi, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1011, 163–170 CrossRef CAS PubMed.
- C. F. Clavijo, K. L. Hoffman, J. J. Thomas, B. Carvalho, L. F. Chu, D. R. Drover, G. B. Hammer, U. Christians and J. L. Galinkin, Anal. Bioanal. Chem., 2011, 400, 715–728 CrossRef CAS PubMed.
- Y. Yamini, A. Pourali, S. Seidi and M. Rezazadeh, Anal. Methods, 2014, 6, 5554–5565 RSC.
- T. A. Isbell, E. C. Strickland, J. Hitchcock, G. McIntire and C. L. Colyer, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2015, 980, 65–71 CrossRef CAS PubMed.
- C. Cruces-Blanco and A. M. García-Campaña, TrAC, Trends Anal. Chem., 2012, 31, 85–95 CrossRef CAS.
- P. S. Francis, J. L. Adcock, J. W. Costin, S. D. Purcell, F. M. Pfeffer and N. W. Barnett, J. Pharm. Biomed. Anal., 2008, 48, 508–518 CrossRef CAS PubMed.
- J. A. Murillo Pulgarín, L. F. García Bermejo and M. N. Sánchez García, Anal. Chim. Acta, 2007, 602, 66–74 CrossRef PubMed.
- J. A. M. Pulgarín, L. F. G. Bermejo, J. M. L. Gallego and M. N. S. García, Talanta, 2008, 74, 1539–1546 CrossRef PubMed.
- A. Babaei and M. Babazadeh, Anal. Methods, 2011, 3, 2400–2405 RSC.
- E. Alipour and S. Gasemlou, Anal. Methods, 2012, 4, 2962–2969 RSC.
- J. Moros, N. Galipienso, R. Vilches, S. Garrigues and M. d. l. Guardia, Anal. Chem., 2008, 80, 7257–7265 CrossRef CAS PubMed.
- Y. Ya, W. Xiaoshu, D. Qing, J. Lin and T. Yifeng, Anal. Methods, 2015, 7, 4502–4507 RSC.
- A. Sheibani, M. R. Shishehbore and E. Mirparizi, Spectrochim. Acta, Part A, 2010, 77, 535–538 CrossRef CAS PubMed.
- M. Amjadi, J. L. Manzoori and J. Hassanzadeh, Anal. Lett., 2009, 42, 1539–1551 CrossRef CAS.
- M. Kolaei, K. Dashtian, Z. Rafiee and M. Ghaedi, Ultrason. Sonochem., 2016, 33, 240–248 CrossRef CAS PubMed.
- M. Sarwar and T. Aman, Microchem. J., 1984, 30, 304–309 CrossRef CAS.
- D. K. Singh and A. Sahu, J. Chin. Chem. Soc., 2005, 52, 247–251 CrossRef CAS.
- G. Sakai, K. Ogata, T. Uda, N. Miura and N. Yamazoe, Sens. Actuators, B, 1998, 49, 5–12 CrossRef CAS.
- M. Iranifam, TrAC, Trends Anal. Chem., 2016, 80, 387–415 CrossRef CAS.
- Y. Su, Y. Xie, X. Hou and Y. Lv, Appl. Spectrosc. Rev., 2014, 49, 201–232 CrossRef CAS.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- A. Sarafraz Yazdi and Z. Es'haghi, J. Chromatogr. A, 2005, 1094, 1–8 CrossRef CAS PubMed.
- M. Moller, K. Aleksa, P. Walasek, T. Karaskov and G. Koren, Forensic Sci. Int., 2010, 196, 64–69 CrossRef CAS PubMed.
- W. Z. Shou, M. Pelzer, T. Addison, X. Jiang and W. Naidong, J. Pharm. Biomed. Anal., 2002, 27, 143–152 CrossRef CAS PubMed.
- Q. Li, M. H. W. Lam, R. S. S. Wu and B. Jiang, J. Chromatogr. A, 2010, 1217, 1219–1226 CrossRef CAS PubMed.
- F. Augusto, L. W. Hantao, N. G. S. Mogollón and S. C. G. N. Braga, TrAC, Trends Anal. Chem., 2013, 43, 14–23 CrossRef CAS.
- L. Qin, X. W. He, W. Y. Li and Y. K. Zhang, J. Chromatogr. A, 2008, 1187, 94–102 CrossRef CAS PubMed.
- X. Song, J. Li, S. Xu, R. Ying, J. Ma, C. Liao, D. Liu, J. Yu and L. Chen, Talanta, 2012, 99, 75–82 CrossRef CAS PubMed.
- Y. Wen, L. Chen, J. Li, D. Liu and L. Chen, TrAC, Trends Anal. Chem., 2014, 59, 26–41 CrossRef CAS.
- A. Andrade-Eiroa, M. Canle, V. Leroy-Cancellieri and V. Cerdà, TrAC, Trends Anal. Chem., 2016, 80, 641–654 CrossRef CAS.
- C. Herrero-Latorre, J. Barciela-García, S. García-Martín, R. M. Peña-Crecente and J. Otárola-Jiménez, Anal. Chim. Acta, 2015, 892, 10–26 CrossRef CAS PubMed.
- M. Á. González-Curbelo, A. V. Herrera-Herrera, J. Hernández-Borges and M. Á. Rodríguez-Delgado, J. Sep. Sci., 2013, 36, 556–563 CrossRef PubMed.
- Y. Wen, Z. Niu, Y. Ma, J. Ma and L. Chen, J. Chromatogr. A, 2014, 1368, 18–25 CrossRef CAS PubMed.
- B. S. Sherigara, W. Kutner and F. D'Souza, Electroanalysis, 2003, 15, 753–772 CrossRef CAS.
- J. Ma, R. Xiao, J. Li, J. Yu, Y. Zhang and L. Chen, J. Chromatogr. A, 2010, 1217, 5462–5469 CrossRef CAS PubMed.
- L. M. Ravelo-Pérez, A. V. Herrera-Herrera, J. Hernández-Borges and M. Á. Rodríguez-Delgado, J. Chromatogr. A, 2010, 1217, 2618–2641 CrossRef PubMed.
- E. Lee, D.-W. Park, J.-O. Lee, D. S. Kim, B. H. Lee and B. S. Kim, Colloids Surf., A, 2008, 313–314, 202–206 Search PubMed.
- X. Kan, Y. Zhao, Z. Geng, Z. Wang and J.-J. Zhu, J. Phys. Chem. C, 2008, 112, 4849–4854 CAS.
- L. Chen, X. Wang, W. Lu, X. Wu and J. Li, Chem. Soc. Rev., 2016, 45, 2137–2211 RSC.
- S. Xu, J. Li and L. Chen, J. Mater. Chem., 2011, 21, 4346–4351 RSC.
- Y. M. Oskoei, H. Fattahi, J. Hassanzadeh and A. M. Azar, Anal. Sci., 2016, 32, 193–199 CrossRef CAS PubMed.
- Z. Lin, W. Xue, H. Chen and J.-M. Lin, Chem. Commun., 2012, 48, 1051–1053 RSC.
- C. M. Hindson, P. S. Francis, G. R. Hanson, J. L. Adcock and N. W. Barnett, Anal. Chem., 2010, 82, 4174–4180 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Absorption and fluorescence spectra of synthesized carbon dots, all optimization graphs and table for interfering studies. See DOI: 10.1039/c6ra22074f |
| This journal is © The Royal Society of Chemistry 2016 |