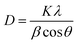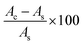Antimicrobial, antioxidant and anticancer activity of biogenic silver nanoparticles – an experimental report†
S. Bhakya‡
*a,
S. Muthukrishnan‡b,
M. Sukumarana,
M. Grijalvac,
L. Cumbalc,
J. H. Franklin Benjamind,
T. Senthil Kumare and
M. V. Raob
aP.G. & Research Department of Zoology, Rajah Serfoji Govt. College (Autonomous), Thanjavur-613 005, Tamil Nadu, India. E-mail: bhakya19@gmail.com
bDepartment of Plant Science, Bharathidasan University, Tiruchirappalli-620 024, Tamil Nadu, India. E-mail: muthukrishnan1985@gmail.com
cCentro de Nanociencia y Nanotecnología, Universidad de las Fuerzas Armadas ESPE, PO Box: 171-5-231B, Sangolqui, Ecuador
dBotanical Survey of India, Southren Regional Centre, Coimbatore-641 003, India
eDepartment of Industry University Collaboration, Bharathidasan University, Tiruchirappalli-620 024, Tamil Nadu, India
First published on 15th August 2016
Abstract
In the present study, use of a Helicteres isora stem bark extract for the biosynthesis of AgNPs is described. It was observed that the aqueous silver (Ag+) ions, once associated in the stem bark extract, were reduced in solution, thereby leading to formation of the stable AgNPs. These AgNPs were characterized using several techniques. The nanoparticles show a maximum absorbance at 419–431 nm in the ultraviolet-visible spectra. The presence of the steroid sapogenin was identified using Fourier transform-infrared (FTIR) spectroscopy. The reduction of the Ag+ ions to elemental silver was investigated by using X-ray diffraction (XRD). Transmission electron microscopy (TEM) revealed the formation of monodisperse, with low polydispersity, nanoparticles of 25.55 nm, and the presence of elemental silver was confirmed through energy dispersive spectroscopy (EDX) analysis. The AgNPs showed antioxidant activities such as DPPH, hydrogen peroxide and nitric oxide radical scavenging and a reducing power compared to the standard compounds. The antibacterial effect was determined against test strains, showing significant inhibition. The antiproliferative activity of the AgNPs was demonstrated using oral carcinoma (KB) cells with MTT, and confirmed using AO/EtBr, comet assay, DCFH-DA and Rhodamine 123 staining. In the toxicity study, a significant mortality rate was observed against Artemia with an IC50 concentration of 70 μg mL−1 and 108 h exposure. The NPs showed as cytotoxic against Artemia at 108 h, so they are cytotoxic at high concentrations and after prolonged exposure.
1. Introduction
Worldwide cancer is the leading cause of death. In India about 6% of deaths are due to cancer, which contributes 8% of the global cancer mortality. Oral cancer is in India the third and worldwide the eleventh most common cancer with over 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new cases annually, and over 30% of all cancers and 90% of oral cancers have been caused by tobacco use and excessive alcohol consumption.1 All of the tobacco consumption developed oral cancers have annual incidence rates ranging from 5.2/1000 to 30.2/1000, whereas non-users develop the fewest oral lesions with incidence rates from 0.6/1000. An increasing number of teenagers are being affected and 25% of the cases have no associated risk factors.2 For the treatment of oral cancer, some possible methods are surgery, radiation therapy, chemotherapy, targeted therapy and palliative treatment but the cost is high. So, in the 21st century, nanotechnology is an emerging technology and it has incredible applications in medicine, drug delivery,3 molecular imaging,4 wound healing,5 the diagnosis of cardiovascular diseases,6 antibacterial processes,7,8 photocatalytic degradation9,10 and amperometry.11 The major interest has been the development of new particles and investigation of their properties by fine-tuning the particle shape, size and distribution.
000 new cases annually, and over 30% of all cancers and 90% of oral cancers have been caused by tobacco use and excessive alcohol consumption.1 All of the tobacco consumption developed oral cancers have annual incidence rates ranging from 5.2/1000 to 30.2/1000, whereas non-users develop the fewest oral lesions with incidence rates from 0.6/1000. An increasing number of teenagers are being affected and 25% of the cases have no associated risk factors.2 For the treatment of oral cancer, some possible methods are surgery, radiation therapy, chemotherapy, targeted therapy and palliative treatment but the cost is high. So, in the 21st century, nanotechnology is an emerging technology and it has incredible applications in medicine, drug delivery,3 molecular imaging,4 wound healing,5 the diagnosis of cardiovascular diseases,6 antibacterial processes,7,8 photocatalytic degradation9,10 and amperometry.11 The major interest has been the development of new particles and investigation of their properties by fine-tuning the particle shape, size and distribution.
Green nanotechnology is a developing area due to the removal of harmful reagents and it provides an effective synthesis of expected products at a low cost. Noble metal particles exhibit new physicochemical properties that are not observed either in bulk metals or individual molecules. Among the noble metal nanoparticles, silver nanoparticles (AgNPs) in particular are known for their versatile applications in medical industries and defense,12,13 and they are applied spectrally for active camouflage in the military.14 Also, AgNPs were the most efficient as antimicrobials and antioxidants.8,15 Plant extracts have less toxic substances and require minimal purification steps when compared to chemical methods.16 AgNPs have been studied for their capability to diminish microbial infections of the skin and also to prevent bacterial colonization.17 There are several physical and chemical methods employed for the synthesis of AgNPs,18 but these methods have created great environmental issues with the use of toxic substances and the generation of harmful by-products. But, the use of natural entities like microorganisms and plant extracts has been explored for the synthesis of AgNPs and this has been found to be a clean, nontoxic, low cost and eco-friendly method. While using microorganisms has some disadvantages, which include maintaining the cell culture in an aseptic environment, plant mediated synthesis offers several advantages such as a low cost and nontoxic and eco-friendly by-products.19 Plant extracts act as reducing and capping mediators in the synthesis of AgNPs and the particles formed are more stable with different shapes and dimensions, making this a more efficient method than other synthesis methods. Recently, many medicinal plants have been employed for the synthesis of AgNPs such as Cleistanthus collinus,15 Morinda citrifolia,20 Alternanthera sessilis21 and Ceropegia thwaitesii.8
In this paper, we will concentrate our attention on the green synthesis of AgNPs using a Helicteres isora stem bark extract and analysis of their antioxidant and antibacterial activities against human pathogens. Furthermore, the anticancer activity was also determined by investigating their effect on cancer cell viability and measuring the intracellular ROS, apoptotic morphological changes, mitochondrial membrane potential (MMP) changes and DNA damage in oral carcinoma (KB) cells. Also, the present study aims at examining whether AgNPs are toxic to marine brine shrimp, using Artemia salina as a toxicology model organism, and whether the toxicity of AgNPs is related to release of Ag+ from the AgNPs or to specific effects of the AgNPs. The toxic effects of AgNPs on the mortality of Artemia salina are assessed and discussed in the present study.
2. Results and discussion
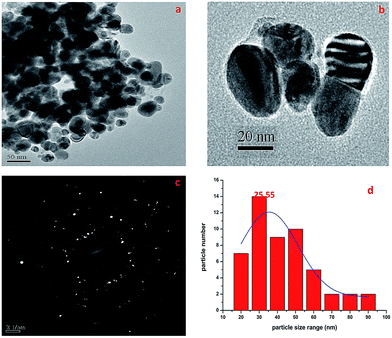
Generally NPs are categorized by their shape, size, surface area, and dispersity. In many applications, these properties of homogeneity are playing a key role.22 When the stem bark extract was combined with AgNO3 and allowed to react at room temperature, within 30 min of reaction the color changed from brown to dark brown (Fig. 1a–c), indicating the formation of AgNPs. It is an efficient and rapid method, which has been very well explained by some investigators who have worked with different plant systems.8,15 During the reaction, the color change was due to the excitation of surface plasmon vibrations in the metal nanoparticles.23 Our results are in agreement with previous report,8 in which the formation of AgNPs within 30 min of incubation is reported.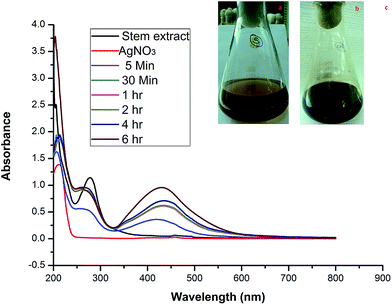 | ||
| Fig. 1 Photographs of (a) the extract and (b) the color change after adding AgNO3. (c) UV-vis spectra of the AgNPs synthesized using stem bark extract after different incubation times. | ||
2.1. Characterization
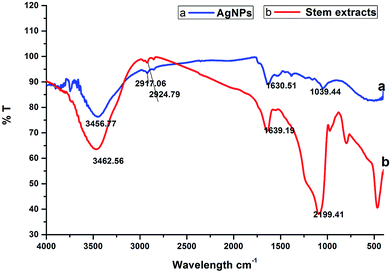
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations, which indicates the presence of alcohol and alkene groups in the extract that may be involved in the reduction process.31 The IR spectrum of the stem/bark extract exhibits a strong band at 1639.19 cm−1 corresponding to a C
C stretching vibrations, which indicates the presence of alcohol and alkene groups in the extract that may be involved in the reduction process.31 The IR spectrum of the stem/bark extract exhibits a strong band at 1639.19 cm−1 corresponding to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (symmetry reduces intensity and NH2 scissoring (1° amines)) stretching mode and this peak is shifted to 1630.51 cm−1, suggesting possible association of the above mentioned groups with the AgNPs synthesis. This amine band indicates that the steroid sapogenin can bind to Ag+ through alcohol and alkene groups.32 This result suggests that the steroid sapogenin is interacting with the AgNPs and also that the secondary structure was not changed during the reaction with Ag+ ions or after binding to the biosynthesized silver nanoparticles.
C (symmetry reduces intensity and NH2 scissoring (1° amines)) stretching mode and this peak is shifted to 1630.51 cm−1, suggesting possible association of the above mentioned groups with the AgNPs synthesis. This amine band indicates that the steroid sapogenin can bind to Ag+ through alcohol and alkene groups.32 This result suggests that the steroid sapogenin is interacting with the AgNPs and also that the secondary structure was not changed during the reaction with Ag+ ions or after binding to the biosynthesized silver nanoparticles.
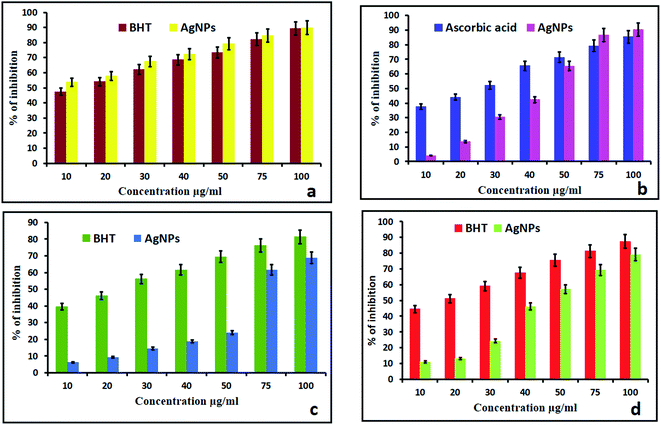
2.2. Antioxidant activity
2.3. Antimicrobial activity
The potential antibacterial activity of the AgNPs was tested against six different pathogenic strains, E. coli, V. cholera, S. typhi, P. aeruginosa, B. subtilis and M. luteus, and compared to a standard antibiotic streptomycin (Fig. 6). The synthesized AgNPs were found to show good antibacterial activity against all six bacterial strains in a dose dependent manner. At a higher (100 μg mL−1) concentration, the AgNPs exhibited a well defined inhibitory effect and produced clearance zones of 10 mm, 5 mm, 8 mm, 3 mm, 5 mm and 12 mm respectively against E. coli, V. cholera, S. typhi, P. aeruginosa, B. subtilis and M. luteus, where the activity was equal to that of streptomycin, which produced clearance zones of 12 mm, 13 mm, 4 mm, 10 mm, 8 mm and 13 mm respectively (Fig. 7). On the other hand, with S. typhi, the AgNPs and streptomycin exhibited 8 mm and 4 mm zones of inhibition respectively at a concentration of 100 μg mL−1. A possible mechanism of the antibacterial activity involves the release of silver cations from the silver nanoparticles that act as reservoirs for the Ag+ bactericidal agent. The interaction of silver cations with bacterial cell membranes, thereby resulting in disturbance of the membrane permeability and of the respiratory system, leads to cell death.43,44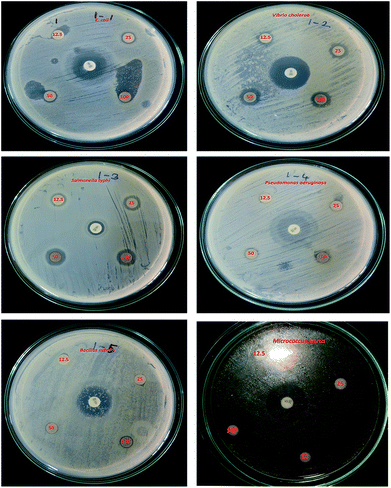 | ||
| Fig. 6 Bactericidal effect against six pathogens, using concentrations of 12.5 μg mL−1, 25 μg mL−1, 50 μg mL−1 and 100 μg mL−1. The standard antibiotic is in the centre. | ||
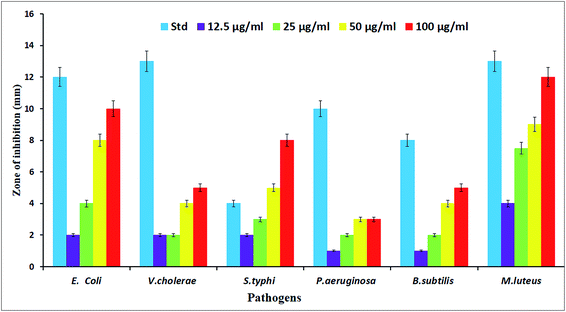 | ||
| Fig. 7 Antimicrobial activity of the synthesized AgNPs against various pathogenic bacterial strains. | ||
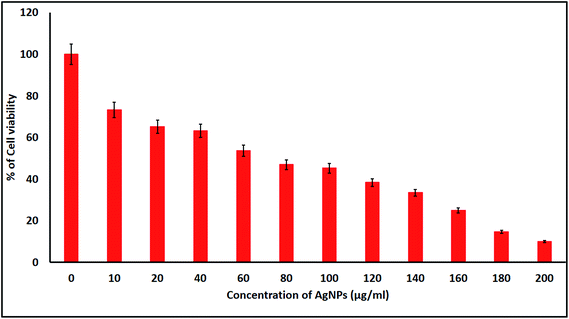
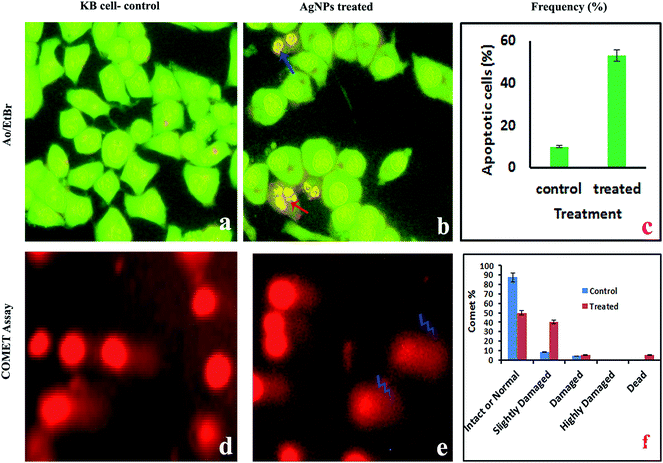
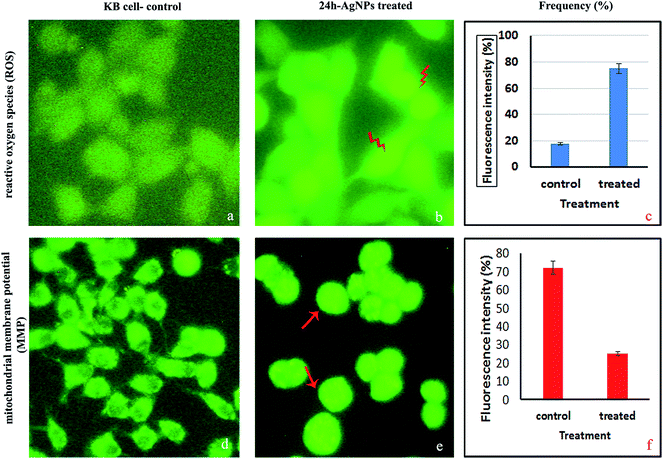
2.4. In vitro anticancer studies
2.5. Cytotoxicity studies
The mortality values are shown in Fig. 11. At 6 h, NP treatment resulted in a 4.23–6.47% mortality rate, with the controls not affected. The mortality rate was dose and exposure time dependent, for a concentration range of 25 μg mL−1 to 200 μg mL−1 and up to 108 h of exposure, with nauplii and adults. At 108 h, the mortality rates of the nauplii and adults were higher for all the concentrations (Fig. 12a–f). The adult mortality rate was less than for the nauplii (data not shown). The mortality rate for the nauplii rose from zero at 25 μg mL−1 to 6.47% at 200 μg mL−1 after 6 h of exposure. These results show that binary compound suspensions of the AgNPs were not acutely toxic to nauplii at high concentrations (75 μg mL−1), after 72 h of exposure. Ates et al.51 reported NPs were not toxic to nauplii at the optimum concentration and with long exposure times.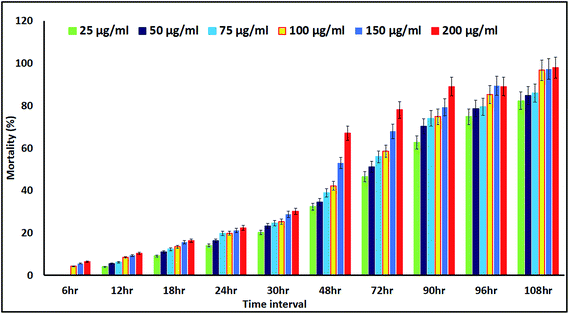 | ||
| Fig. 11 Percentage mortality rates for Artemia nauplii, measured for up to 108 h of exposure to different concentrations of the colloidal biosynthesized AgNPs. | ||
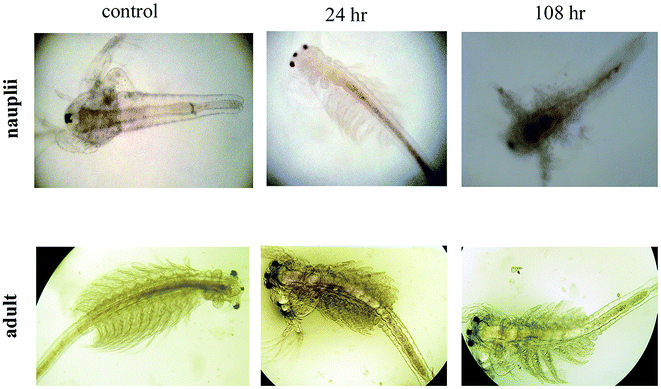 | ||
| Fig. 12 Alteration and accumulation of AgNPs in the Artemia adult and nauplii after 24 h and 108 h of exposure. | ||
3. Materials and methods
3.1. Plant identification and sample preparation
The H. isora plant was collected from the Western Ghats of Tamil Nadu, India and the species were identified and authenticated by Dr J. H. Benjamin Franklin, Scientist-B. Voucher specimens were prepared and deposited at the herbarium of the Botanical Survey of India (BSI), Southern Regional Centre, Coimbatore. After identification, the stem bark material was collected from the collection site, washed with sterile Milli Q water and dried, and then made into a powder. The plant extracts were prepared according to the Bhakya et al.7 method and stored at 4 °C in a refrigerator until further use.3.2. Synthesis of the silver nanoparticles (AgNPs)
Silver nanoparticles were synthesized based on the procedure of Bhakya et al.7 The plant extracts were mixed with a 1 mM aqueous AgNO3 solution in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the mixture was incubated at room temperature for 6 h. Following incubation, the mixture was centrifuged at 18
1 and the mixture was incubated at room temperature for 6 h. Following incubation, the mixture was centrifuged at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min, and after centrifugation the product was washed with Milli Q water and collected into a collecting chamber for air drying.
000 rpm for 20 min, and after centrifugation the product was washed with Milli Q water and collected into a collecting chamber for air drying.
3.3. Characterization of the AgNPs
K – shape factor; λ – X-ray wavelength; β – half the maximum intensity (FWHM) in radians; θ – Bragg’s angle.
3.4. Antibacterial assay
A disc diffusion method was used to determine the antibacterial activity of the biosynthesized AgNPs using the following reference strains: E. coli (ATCC25922), Pseudomonas aeruginosa (ATCC27853), Salmonella typhi (ATCC2426), Vibrio cholera (ATCC2077), Bacillus subtilis (ATCC25923) and Micrococcus luteus (ATCC2077). The overnight inoculated bacterial cultures were spread over freshly prepared Muller-Hinton agar plates. Sterile discs (6 mm, Himedia) containing different concentrations of the silver nanoparticles (12.5, 25, 50 and 100 μg mL−1) were applied and a reference disc of streptomycin was also kept on the plate and incubated at 37 °C for 24 h. The antimicrobial activity of the AgNPs was estimated by measuring the diameter (in millimeters) of the zone of inhibition around the discs after incubation.3.5. In vitro antioxidant assays
The antioxidant activity of the AgNPs (10–100 μg mL−1) was determined using a DPPH assay according to the method introduced by Brand-Williams et al.52 The DPPH free radical scavenging activity of the biosynthesized AgNPs is expressed as % inhibition of DPPH and was calculated using the following formula:where Ac is the control absorbance of the DPPH radicals + methanol; As is the absorbance of the sample/the standard BHT.
The hydrogen peroxide (H2O2) radical scavenging activity of the synthesized AgNPs (10–100 μg mL−1) was quantified using the method devised by Pick and Mizel.53 The total reducing power of the synthesized AgNPs (10–100 μg mL−1) was estimated by following the method devised by Oyaizu.54 The nitric oxide scavenging activity of the synthesized AgNPs (10–100 μg mL−1) was estimated using the Sousa et al.55 reaction method. The obtained results were calculated using the above mentioned formula and compared with the standard antioxidant butylated hydroxytoluene (BHT).
3.6. In vitro anticancer studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well and kept in an incubator for up to 24 h for acclimatization. After 24 h, the cells were treated with various concentrations (10, 20, 40, 60, 80, 100, 120, 140, 160, 180 and 200 μg mL−1) of the AgNPs, along with a control, following a procedure by Dash et al.56 For the cell viability study, the absorbance was measured at 540 nm using a microplate reader. The required concentrations for 50% inhibition of the viability (IC50) were calculated graphically and the optimum dose was used for further studies. Determination of the cell morphology was performed using a phase contrast microscope (Nikon, Eclipse TS100, Japan).
000 cells per well and kept in an incubator for up to 24 h for acclimatization. After 24 h, the cells were treated with various concentrations (10, 20, 40, 60, 80, 100, 120, 140, 160, 180 and 200 μg mL−1) of the AgNPs, along with a control, following a procedure by Dash et al.56 For the cell viability study, the absorbance was measured at 540 nm using a microplate reader. The required concentrations for 50% inhibition of the viability (IC50) were calculated graphically and the optimum dose was used for further studies. Determination of the cell morphology was performed using a phase contrast microscope (Nikon, Eclipse TS100, Japan).3.7. Brine shrimp toxicity study
Brine shrimp (Artemia salina) were hatched using the procedure described by Persoone et al.59 After hatching, counting was performed according to the procedure described by Sorgeloos.60 A study of the cytotoxicity of the AgNPs was conducted using nauplii for 6 h to 108 h. For the experiment, 100 nauplii were placed in 6 well plates, each well containing 20 mL of sea water and a different concentration of the AgNPs (25, 50, 75, 100, 150 and 200 μg mL−1), and were maintained at room temperature for 108 h under light conditions. The mortality rates were then calculated. The experiments were conducted along with a control, and every experiment was carried out in triplicate.4. Conclusion
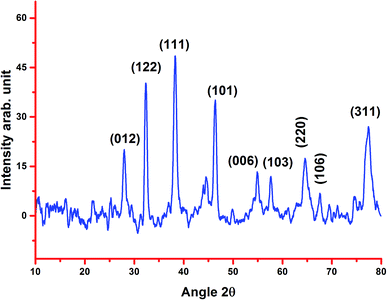
A stable, simple and eco-friendly technique for biosynthesizing AgNPs was effectively established using H. isora stem bark extract. H. isora stem bark contains more phytosterols and saponins, which play major roles as reducing as well as capping agents in the green synthesis of AgNPs. The extract acts as a reducing and stabilizing agent, which was confirmed through FTIR studies. TEM and XRD results revealed that the biosynthesized AgNPs were crystalline in nature with a mean particle size of 25.55 nm. The biosynthesized AgNPs were found to be multifunctional with good antioxidant and antimicrobial activities. The stem bark extract capped AgNP solution showed a significant dose-dependent cytotoxic effect on KB cells. Nauplii showed significant mortality after 108 h of exposure to the AgNPs. Thus, it was concluded that the colloidal AgNPs are not acutely toxic to Artemia. This green synthesis method might be a good alternative to physical and chemical nanosynthesis methods. Hence, this method might be scaled up for industrial production and the manufacturing of ultrafiltration membranes as well as food packaging, and might be of value for many biological and medical applications.Acknowledgements
The work was financially supported by a UGC-Rajiv Gandhi National Fellowship (RGNF) No: F1-17.1/2013-14/RGNF-2013-14-SC-TAM-44942 (SA-III) University Grant Commission New Delhi, India of the first author. We thank the sophisticated analytical instrument facility (SAIF), North-Eastern Hill University (NEHU), Shillong for access to the TEM facility. The authors wish to thank the following individuals who provided valuable advice in the final stage of the revision process: Dr V. Ramalingam, Department of Marine Science, Bharathidasan University, and M. Muthukumar and S. Parthibhan, Research Scholars (Department of Plant Science, Bharathidasan University, Tiruchirappalli-620 024, Tamil Nadu, India), and S. Arul Ganesh, Dr S. Karthikeyeni and S. Arun (Research Scholars, Department of Animal Science, Bharathidasan University, Tiruchirappalli-620 024, India).References
- Z. U. Khan, WebmedCentral CANCER, 2012, 3, WMC003626 Search PubMed.
- R. L. Kruse, J. R. Kramer, G. L. Tyson, Z. Duan, L. Chen, H. B. El-Serag and F. Kanwal, Hepatology, 2014, 60, 1871–1878 CrossRef PubMed.
- C. G. Kumar and Y. Poornachandra, Colloids Surf., B, 2015, 125, 110–119 CrossRef CAS PubMed.
- J. Sekuła, J. Nizioł, W. Rode and T. Ruman, Anal. Chim. Acta, 2015, 875, 61–72 CrossRef PubMed.
- T. Gunasekaran, T. Nigusse and M. D. Dhanaraju, J. Am. Coll. Clin. Wound Spec., 2011, 3, 82–96 CrossRef PubMed.
- B. Godin, J. H. Sakamoto, R. E. Serda, A. Grattoni, A. Bouamrani and M. Ferrari, Trends Pharmacol. Sci., 2010, 31, 199–205 CrossRef CAS PubMed.
- S. Bhakya, S. Muthukrishnan, M. Sukumaran and M. Muthukumar, Appl. Nanosci., 2015, 1–12 CAS.
- S. Muthukrishnan, S. Bhakya, T. S. Kumar and M. Rao, Ind. Crops Prod., 2015, 63, 119–124 CrossRef CAS.
- S. Bhakya, S. Muthukrishnan, M. Sukumaran, M. Muthukumar, S. Kumar and M. Rao, J. Biorem. Biodegrad., 2015, 6, 312 Search PubMed.
- T. Sinha, M. Ahmaruzzaman and A. Bhattacharjee, J. Environ. Chem. Eng., 2014, 2, 2269–2279 CrossRef CAS.
- A. C. Joshi, G. B. Markad and S. K. Haram, Electrochim. Acta, 2015, 161, 108–114 CrossRef CAS.
- M. El-Rafie, T. I. Shaheen, A. Mohamed and A. Hebeish, Carbohydr. Polym., 2012, 90, 915–920 CrossRef CAS PubMed.
- M. Jalali, S. Dauterstedt, A. Michaud and R. Wuthrich, Composites, Part B, 2011, 42, 1420–1426 CrossRef.
- E. Wetzel, http://nano--tech.blogspot.in/p/military.html, 2015.
- N. Kanipandian, S. Kannan, R. Ramesh, P. Subramanian and R. Thirumurugan, Mater. Res. Bull., 2014, 49, 494–502 CrossRef CAS.
- A. Melaiye and W. J. Youngs, Expert Opin. Ther. Pat., 2005, 15, 125–130 CrossRef CAS.
- D. G. Maki, Clin. Infect. Dis., 2010, 50, 1580–1587 CrossRef PubMed.
- M. Zargar, K. Shameli, G. R. Najafi and F. Farahani, J. Ind. Eng. Chem., 2014, 20, 4169–4175 CrossRef CAS.
- R. Arunachalam, S. Dhanasingh, B. Kalimuthu, M. Uthirappan, C. Rose and A. B. Mandal, Colloids Surf., B, 2012, 94, 226–230 CrossRef CAS PubMed.
- G. Sathishkumar, C. Gobinath, K. Karpagam, V. Hemamalini, K. Premkumar and S. Sivaramakrishnan, Colloids Surf., B, 2012, 95, 235–240 CrossRef CAS PubMed.
- K. Niraimathi, V. Sudha, R. Lavanya and P. Brindha, Colloids Surf., B, 2013, 102, 288–291 CrossRef CAS PubMed.
- H. Jiang, K.-s. Moon, Z. Zhang, S. Pothukuchi and C. Wong, J. Nanopart. Res., 2006, 8, 117–124 CrossRef CAS.
- A. Ahmad, P. Mukherjee, S. Senapati, D. Mandal, M. I. Khan, R. Kumar and M. Sastry, Colloids Surf., B, 2003, 28, 313–318 CrossRef CAS.
- V. Ramalingam, R. Rajaram, C. PremKumar, P. Santhanam, P. Dhinesh, S. Vinothkumar and K. Kaleshkumar, J. Basic Microbiol., 2014, 54, 928–936 CrossRef CAS PubMed.
- M. Kowshik, N. Deshmukh, W. Vogel, J. Urban, S. K. Kulkarni and K. Paknikar, Biotechnol. Bioeng., 2002, 78, 583–588 CrossRef CAS PubMed.
- R. Mata, J. R. Nakkala and S. R. Sadras, Colloids Surf., B, 2015, 128, 276–286 CrossRef CAS PubMed.
- C. Krishnaraj, E. Jagan, S. Rajasekar, P. Selvakumar, P. Kalaichelvan and N. Mohan, Colloids Surf., B, 2010, 76, 50–56 CrossRef CAS PubMed.
- D. E. Sands, Introduction to crystallography, Courier Corporation, 2012 Search PubMed.
- T. J. I. Edison and M. Sethuraman, Process Biochem., 2012, 47, 1351–1357 CrossRef CAS.
- T. Prathna, N. Chandrasekaran and A. Mukherjee, Colloids Surf., A, 2011, 390, 216–224 CrossRef CAS.
- J. Kasthuri, S. Veerapandian and N. Rajendiran, Colloids Surf., B, 2009, 68, 55–60 CrossRef CAS PubMed.
- A. M. Fayaz, K. Balaji, M. Girilal, R. Yadav, P. T. Kalaichelvan and R. Venketesan, Nanomedicine: Nanotechnology, Biology and Medicine, 2010, 6, 103–109 CrossRef CAS PubMed.
- T. Suman, S. R. Rajasree, A. Kanchana and S. B. Elizabeth, Colloids Surf., B, 2013, 106, 74–78 CrossRef CAS PubMed.
- M. R. Mozafari, J. Flanagan, L. Matia-Merino, A. Awati, A. Omri, Z. E. Suntres and H. Singh, J. Sci. Food Agric., 2006, 86, 2038–2045 CrossRef CAS.
- W. He, Y.-T. Zhou, W. G. Wamer, M. D. Boudreau and J.-J. Yin, Biomaterials, 2012, 33, 7547–7555 CrossRef CAS PubMed.
- E.-J. Yang, S. Kim, J. S. Kim and I.-H. Choi, Biomaterials, 2012, 33, 6858–6867 CrossRef CAS PubMed.
- A. Gibson, Eur. J. Pharmacol., 2001, 411, 1–10 CrossRef CAS PubMed.
- D. Rees, R. Palmer and S. Moncada, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 3375–3378 CrossRef CAS.
- D. S. Bredt, P. M. Hwang and S. H. Snyder, Nature, 1990, 347, 768–770 CrossRef CAS PubMed.
- N. J. Miller, C. Castelluccio, L. Tijburg and C. Rice-Evans, FEBS Lett., 1996, 392, 40–44 CrossRef CAS PubMed.
- I. Emerit, Free Radicals Biol. Med., 1994, 16, 99–109 CrossRef CAS PubMed.
- Y.-L. Lin, I.-M. Juan, Y.-L. Chen, Y.-C. Liang and J.-K. Lin, J. Agric. Food Chem., 1996, 44, 1387–1394 CrossRef CAS.
- X.-x. Dong, Y. Wang and Z.-h. Qin, Acta Pharmacol. Sin., 2009, 30, 379–387 CrossRef CAS PubMed.
- E. Paszek, J. Czyz, O. Woźnicka, D. Jakubiak, J. Wojnarowicz, W. Łojkowski and E. Stępień, J. Biomed. Nanotechnol., 2012, 8, 957–967 CrossRef CAS PubMed.
- K. Renugadevi and R. V. Aswini, Int. J. Nanomater. Biostruct., 2012, 2, 5–10 Search PubMed.
- R. Vivek, R. Thangam, K. Muthuchelian, P. Gunasekaran, K. Kaveri and S. Kannan, Process Biochem., 2012, 47, 2405–2410 CrossRef CAS.
- P. D. Ray, B.-W. Huang and Y. Tsuji, Cell. Signalling, 2012, 24, 981–990 CrossRef CAS PubMed.
- M. Jeyaraj, M. Rajesh, R. Arun, D. MubarakAli, G. Sathishkumar, G. Sivanandhan, G. K. Dev, M. Manickavasagam, K. Premkumar and N. Thajuddin, Colloids Surf., B, 2013, 102, 708–717 CrossRef CAS PubMed.
- P. M. G. Nair, S. Y. Park and J. Choi, Chemosphere, 2013, 92, 592–599 CrossRef CAS PubMed.
- A. Jones, J. Van Blerkom, P. Davis and A. A. Toledo, Hum. Reprod., 2004, 19, 1861–1866 CrossRef PubMed.
- M. Ates, J. Daniels, Z. Arslan and I. O. Farah, Environ. Monit. Assess., 2013, 185, 3339–3348 CrossRef CAS PubMed.
- W. Brand-Williams, M.-E. Cuvelier and C. Berset, LWT--Food Sci. Technol., 1995, 28, 25–30 CrossRef CAS.
- E. Pick and D. Mizel, J. Immunol. Methods, 1981, 46, 211–226 CrossRef CAS PubMed.
- M. Oyaizu, Jpn. J. Nutr., 1986, 44, 307–315 CrossRef CAS.
- A. Sousa, I. C. Ferreira, L. Barros, A. Bento and J. A. Pereira, LWT--Food Sci. Technol., 2008, 41, 739–745 CrossRef CAS.
- S. K. Dash, S. Chattopadhyay, T. Ghosh, S. Tripathy, S. Das, D. Das and S. Roy, ISRN Oncol., 2013, 2013, 709269 Search PubMed.
- N. P. Singh, M. T. McCoy, R. R. Tice and E. L. Schneider, Exp. Cell Res., 1988, 175, 184–191 CrossRef CAS PubMed.
- A. Roy, A. Ganguly, S. BoseDasgupta, B. B. Das, C. Pal, P. Jaisankar and H. K. Majumder, Mol. Pharmacol., 2008, 74, 1292–1307 CrossRef CAS PubMed.
- G. Persoone, A. Van de Vel, M. Van Steertegem and B. De Nayer, Aquat. Toxicol., 1989, 14, 149–167 CrossRef CAS.
- P. Sorgeloos, Mar. Ecol.: Prog. Ser., 1980, 3, 363–364 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17569d |
| ‡ Two authors equally contributed. |
| This journal is © The Royal Society of Chemistry 2016 |