Enhanced photocatalytic performance and morphology evolvement of PbWO4 dendritic nanostructures through Eu3+ doping†
Abstract
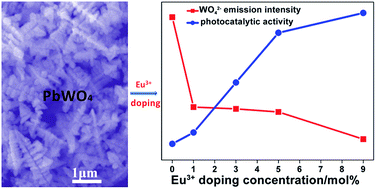
Eu3+ doped PbWO4 dendritic nanostructures have been prepared by a facile hydrothermal technique and characterized by XRD, SEM, TEM, XPS and PL spectroscopy. The results indicate that the changing of the Eu3+ doping concentration in a reasonable range can adjust the morphology, and has no obvious effect on the phase structure of the obtained materials. Moreover, the Eu3+ doping can lead to the enhancement of photocatalytic activity of PbWO4. Compared with pure PbWO4 dendritic nanostructures, the PbWO4:x%Eu3+ samples exhibit improved photocatalytic activity in the decomposition of Rhodamine B (RhB). The enhanced performance of the photocatalyst might be ascribed to efficient separation of electron and hole pairs after doping Eu3+ into the PbWO4 lattice.

 Please wait while we load your content...
Please wait while we load your content...