 Open Access Article
Open Access ArticleAqueous self-assembly of short hydrophobic peptides containing norbornene amino acid into supramolecular structures with spherical shape†
Alessandro Ruffoni*a,
Maria V. Cavannab,
Simona Argentiereb,
Silvia Locarnoa,
Sara Pellegrinoa,
Maria Luisa Gelmia and
Francesca Clerici*a
aUniversità degli Studi di Milano, Dipartimento di Scienze Farmaceutiche, Sezione di Chimica Generale e Organica “Alessandro Marchesini”, Via Venezian 21, 20133 Milano, Italy. E-mail: alessandro.ruffoni@irbbarcelona.org; francesca.clerici@unimi.it; Fax: +39 0250314476; Tel: +39 0250314472
bFondazione Filarete, V. Ortles 22, Milano, Italy
First published on 16th September 2016
Abstract
The preparation and self-assembly of short hydrophobic peptides able to solubilize in water through the formation of supramolecular assembly is reported. The two diastereoisomeric pentapeptides AcAla-NRB-Ala-Aib-AlaNH2 1 and 2 containing the two enantiomers of non-proteinogenic norbornene amino acid (NRB) were synthesized in an efficient way and in good yields. They were insoluble in organic solvent, except MeOH and DMSO, but completely soluble in water despite that they are made of hydrophobic amino acids. The formation of a supramolecular assembly in water was assessed by Transmission Electron Microscopy (TEM) and Dynamic Light Scattering (DLS) using 1 and 2 individually or in a mixture. Conformational analysis on the two diastereoisomers, performed in CD3CN, CD3OH and in H2O/D2O, indicated the formation of a stable 310-helix structure for both peptides: the helix structure is more stable in CD3CN and CD3OH than in H2O/D2O where a helix/random coil transition was observed. Apparently, the norbornene moiety plays a role in the stabilization, in fact 1R2R3R-norbornene AA present in peptide 2 induces a more stable secondary structure with respect to the 1S2S3S-isomer present in peptide 1.
Introduction
Self-assembly is a spontaneous process by which unordered systems of monomers organize into ordered structures as the result of non-covalent interactions including van der Waals, electrostatic, hydrogen bonding, and stacking interactions.1 Self-assembly lies behind a number of biological nanostructures such as DNA double helix, protein's tertiary or quaternary structure, cell membranes upon self-assembly of phospholipids. In this scenario, better insight into the formation of vesicle-like supramolecular structures from small molecules, especially simple peptides, is of particular interest for the understanding of prebiotic organization and life.2 Moreover, the concept of self-assembly has also been used in many disciplines for constructing useful materials. In particular, a number of papers report on spontaneous assembly of peptides into ordered nanostructures with a variety of morphologies and the number is still increasing.3 Most of them describe supramolecular assembly from high molecular weight peptides as well as by small- or medium size peptides conjugated with molecules of nonpeptidic nature. Short peptides alone self-organize predominantly into nanotubes and nanofibers.4 Spherical (micellar and vesicle-like) architectures are less described although their appear very attractive, due to their promising applications in biomedicine and nanotechnology.3c,3m,5 Besides the numerous advantages of using peptides, some limitations are well-known such as a low stability in biological medium and their unstable conformation especially when they are short or medium-sized. The insertion of unnatural amino acids in the peptide sequences is a well-known tool to overcome these problems.In particular, the introduction of Cα-tetrasubstituted α-amino acid residues into peptide backbones generates models characterized by reduced conformational flexibility, high tendency to adopt predetermined secondary structures, and enhanced metabolic stability.6 Both theoretical and experimental studies on this subject have been published and the group of Cα-tetrasubstituted residues in which the quaternary α-carbon forms part of a ring has been the object of extensive investigation.7 Moreover, the use of non-proteinogenic amino acids allows an expansion in terms of structural diversification.8 Notwithstanding this interest, studies on the self-assembly of short peptides containing cyclic Cα-tetrasubstituted amino acids are very rare.9
Herein we report on the preparation of two diastereoisomeric pentapeptides AcAla-NRB-Ala-Aib-AlaNH2 1 and 2, containing the two enantiomers of the unnatural constrained norbornene amino acid (NRB) 3 (Fig. 1). Although not derived from natural sources the bicycloheptane scaffold is known to possess very interesting features.10a,b The norbornane amino acid is known to act as an inhibitor of System L-Amino Acid Transporter and several studies were undertaken concerning its ability to suppress cancer cells proliferation.11a,b On the other hand the presence of the norbornene scaffold allows an efficient labeling of peptides and proteins.12
Despite the hydrophobic nature of the amino acids, the two peptides 1 and 2 resulted completely soluble in water due to formation of supramolecular assemblies of spherical shape.
The formation of supramolecular assembly in water was assessed by Transmission Electron Microscopy (TEM) and Dynamic Light Scattering (DLS) using 1 and 2 individually or in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture. Taking into account the growing interest toward designed peptide-based nanoparticles as candidates for the controlled delivery of drugs, proteins, genes and nucleotides, the stability of the obtained assemblies in blood serum was also studied. Experimental results showed that the supramolecular assembly of peptide 1 and 2 in mixture was stable in serum at 37 °C with unmodified dimension with respect to water.
1 mixture. Taking into account the growing interest toward designed peptide-based nanoparticles as candidates for the controlled delivery of drugs, proteins, genes and nucleotides, the stability of the obtained assemblies in blood serum was also studied. Experimental results showed that the supramolecular assembly of peptide 1 and 2 in mixture was stable in serum at 37 °C with unmodified dimension with respect to water.
Results and discussion
Peptide synthesis
The synthesis of the designed peptides 1 and 2 is reported in Scheme 1. Norbornene nitroesters 4 and 5 were prepared on a gram scale using a modified multicomponent protocol [ethyl nitroacetate (1 eq.), formaldehyde (37%, 5 eq.), cyclopentadiene (5 eq.), acetic acid (5 eq.), 50 °C, THF, 12 h] described by Carrol13 in 88% yields (ratio: 15/85). 4 and 5 were reduced with an excess of Zn dust in H3PO4 (1 M in THF) affording the amines 6 and 7 (80% yields) that were easily separated by flash chromatography. Pure compound 8 (90% overall yields) was obtained through hydrolysis of ester 7 (KOH 6 eq., MeOH, 70 °C for 24 h, then HCl/MeOH, 0 °C) and Boc protection (Boc2O, K2CO3, H2O/MeOH) and purified by precipitation from reaction mixture with HCl 1 M at 0 °C. 8 was successfully coupled using a standard protocol (HOAt 1.1 eq., EDC 1.1 eq., DIPEA 2.2 eq., DCM) with the trifluoroacetic salt of the peptide 9 (H-Ala-Aib-Ala-NH2). This last was previously prepared by a linear Boc chemistry solution strategy recently reported by us (50% overall yield on a gram scale).10bThe Boc protected tetrapeptides 10 and 11 were purified by precipitation in DCM/n-hexane in satisfactory yield (85%) as an inseparable diastereoisomeric mixture. The deprotection of 10/11 mixture was attempted with TFA but the recovery of the free amines was very troublesome due to their high water solubility. On the other hand the microwave assisted Boc deprotection (150 °C, 20 min, 80 watt)14 of tetrapeptides 10/11 afforded the corresponding amines 12 and 13 (91% yield). The diastereoisomeric mixture of 12 and 13 was then condensed with Ac-Ala-OH. To avoid the high trend of racemization of the acetylated alanine under standard coupling condition, a different protocol, recently assessed by our group, was tested consisting in the use of EEDQ, in absence of base, at the lowest temperature reachable by a conventional compressed air-cooled MW reactor (60 °C, 80 watt).10b Diastereoisomeric pentapeptides 1 and 2 were precipitated from the reaction mixture in pure form with Et2O (88% yield) and separated by RP-HPLC (see ESI† for details). Taking in account future biomedical applications of the mixture of peptide 1 and 2 It is noteworthy that entire protocol required just one chromatographic purification steps at the level of amino acids 6 and 7.
Morphology and size of supramolecular assemblies of peptides in water
The behavior of 1 and 2 in aqueous environment was investigated by dissolving them individually or in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture at the concentration of 5.0 mg mL−1 in water. The self-assembly of 1 and 2 was firstly assessed by Dynamic Light Scattering (DLS) that represents a valuable tool to measure the size of particles in the sub-micron region. The DLS profiles of 1 and 2 as well as their mixture are reported in Fig. 2.
1 mixture at the concentration of 5.0 mg mL−1 in water. The self-assembly of 1 and 2 was firstly assessed by Dynamic Light Scattering (DLS) that represents a valuable tool to measure the size of particles in the sub-micron region. The DLS profiles of 1 and 2 as well as their mixture are reported in Fig. 2.
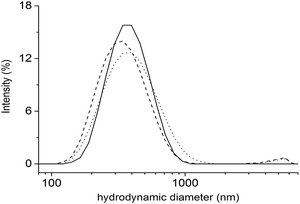 | ||
Fig. 2 DLS results. Size distribution by intensity of 1 (straight) and 2 (dash) oligopeptides, as well as their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture (dot). Mean results are given for three different measurements. 1 mixture (dot). Mean results are given for three different measurements. | ||
All the tested samples showed the capability to self-assemble in aqueous environment and form aggregates whose hydrodynamic diameter values (i.e. mean size) ranged from about 320 to 370 nm (Fig. 2). Although the measured size values were quite similar, 1 formed supramolecular structures (357.0 nm) slightly bigger than 2 (322.9 nm). Further, as shown in Fig. 2, the obtained assemblies showed monomodal distributions with polydispersity index (pdI) of 0.117 and 0.195 for 1 and 2, respectively. When oligopeptides 1 and 2 were mixed together, monodisperse structures were still detected by DLS (pdI 0.185), whose size (369.1 nm) was similar to that obtained by individual oligopeptides. Accordingly, further characterization was done on the mixture of 1 and 2. All the DLS measurements were run on freshly prepared oligopeptide solutions and the formation of self-assembled supramolecular structures in water was found to be fully reproducible.
In order to be used as drug delivery carriers, nanoparticles should remain intact and circulate in the blood for a sufficiently long period of time after intravenous injection to accumulate at the target site.15 The stability of the oligopeptide assemblies (mixture of 1 and 2) in a complex medium mimicking the in vivo conditions, namely fetal bovine serum (FBS), was evaluated and tested by DLS (Fig. 3).
As a control, pure FBS was analyzed by DLS and showed two peaks due to its protein composition. Interestingly, the peak of the supramolecular assemblies of 1 and 2 in mixture in pure FBS was clearly visible. This suggested that the interaction with FBS proteins did not affect the overall structure of the supramolecular assemblies which appeared stable under physiological mimicking conditions without substantial changes in their size. To further investigate the morphology of the supramolecular assemblies, the mixture of 1 and 2 was analyzed by TEM. Notably, the assemblies showed spherical shape with diameters ranging from 30 to 65 nm (Fig. 4).
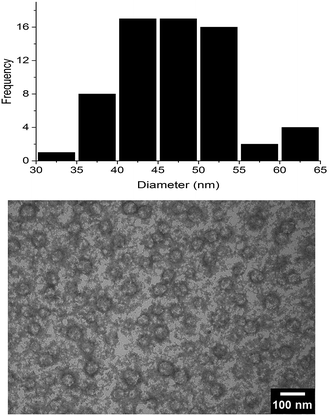 | ||
Fig. 4 TEM micrograph of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of oligopeptides 1 and 2 with uranyl acetate as negative staining agent. 1 mixture of oligopeptides 1 and 2 with uranyl acetate as negative staining agent. | ||
The discrepancy between TEM and DLS data could arise from the formation of agglomerates (i.e. weakly bound aggregates) of the assemblies in suspension to minimize interfacial energy. Finally, the critical aggregation concentrations (CACs) of 1 and 2 in aqueous solution were determined by light scattering.16 To this aim, different samples with increasing concentration of either 1 or 2 were prepared and their count rate (i.e. the intensity of scattered light in DLS) was monitored (see ESI, Tables TS7 and TS8†). According to the experimental data, the count rate was found to increase with increasing the concentration of either 1 or 2. However, samples with concentration higher than 5.0 and 26.0 mg mL−1 for 1 and 2, respectively, led to a decrease in the count rate value. This suggested that the assemblies' concentration increased up to an optimal value and then decreased, probably due to altered interactions between the involved oligopeptides.
NMR analysis
NMR experiments (1H, 13C, COSY, NOESY, ROESY, HSQC, HMBC) in CD3OH and H2O/D2O were performed at the concentration of 16 mg mL−1 (when not specified differently) and allowed the complete characterization of 1 and 2.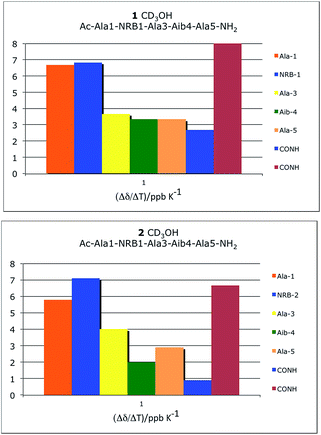 | ||
| Fig. 5 Temperature dependence of amide chemical shift (Δδ/ΔT 273–300 K). Analyses in CD3OH for peptide 1 (32 mg mL−1) and peptide 2 (26 mg mL−1). | ||
A different behaviour was found in H2O/D2O (Fig. 6) where higher Δδ/ΔT values were detected for all NHs. However, as in the case of CD3OH analyses, peptide 2 shows lower NH values at C-termini (Δδ/ΔT Aib4, Ala5 and NH of the CONH2: 3.8–4.5 ppb K−1), than peptide 1 (Δδ/ΔT Aib4, Ala5 and NH of the CONH2: 4.0–5.5 ppb K−1). Being known that Δδ/ΔT for completely random coil peptide in water is ≈8.0 ppb K−1,19 we hypothesize an equilibrium between helix/random coil structure in H2O/D2O. Moreover is interesting to notice that in water the differences between the two primary amide protons Δδ/ΔT values are smaller than in methanol.20 Especially the second primary amide of peptide 1 with a values of 6 ppb K−1 seems to be involved in a H-bond but we were not able to determinate the inter- or intra-molecular nature of that bound.
| Solvent | 1 MNE ppm δ | 2 MNE ppm δ |
|---|---|---|
| CD3CN | 3.38 | 3.69 |
| CD3OD | 1.94 | 2.79 |
| D2O | 0.25 | 0.85 |
Instead, the absence of a stable helix structure for 1 and a transition helix/random coil structure for 2 were observed in D2O (see ESI† for discussion).
The comparison with MNE values reported in literature22 for differently capped (Ala-Aib)2-Ala pentamers (BocNH, COOMe) corroborates the hypothesis that primary amides at C-terminus together with N-terminal acetyl group strongly stabilized helix conformation in organic solvent.
Finally, long range spatial proximity between NH of Ala5 and specifìc protons of norbornene ring, allows to tentatively assign the absolute stereochemistry of norbornene scaffold (1HNMR in CD3OH; see Fig. SI6†). As a result, the S- and R- stereochemistry was assigned to norbornene scaffold in peptide 1 and 2, respectively.
Taking together all these data, we can affirm that the 1R2R3R-norbornene AA present in peptide 2, induce a more stable 310-helix secondary structure with respect to 1S2S3S-norbornene AA present in peptide 1. For both peptides, the helix structure is more stable in methanol than in water where a helix/random coil transition was observed. Preliminary experiments of concentration dependence of 1H NMR performed in the range of CAC, did not show any change in NH chemical shifts.23 Even if this result can suggest the absence of intermolecular H-bond in aggregates formation process it has to be noted that due to experimental limitation experiment were performed only from 5 mM to 10 mM due to the detection limits.
Conclusions
The two oligopeptides 1 and 2 were synthesized and the experimental data collected in CD3CN and in CD3OD confirmed a conformational preference toward a stable 310-helix structure. Norbornene plays a certain role in such stabilization as observed in a previously published computational model7m and, basing on the magnitude of MNE in CD3CN, it seems to be the best helix inducer among the unnatural constrained amino acids synthesized in our group referring to the same model peptide.6bConformational studies were performed also in H2O/D2O where a helix/random coil transition was observed for both peptides.
Moreover, a deep analysis of the NMR experiments allowed to tentatively assign the absolute stereochemistry to peptide 1 and 2.
Oligopeptides 1 and 2 resulted completely soluble in water due to formation of supramolecular assemblies of spherical shape, despite the hydrophobic nature of the amino acids. Interestingly, the two oligopeptides in mixture gave monodisperse structures similar to that obtained by individual oligopeptides. This result is of high value because allows the availability of a large amount of the self-assembling peptide material useful for several applications. Finally, the stability of the oligopeptide assemblies in serum at 37 °C was tested showing unmodified dimension opening the way to further studies as candidates for drug delivery.
Acknowledgements
This work was partially supported by Ministero dell'Università e della Ricerca (Prin 2010 “Synthesis and biomedical applications of tumor-targeting peptidomimetics” prot. 2010NRREPL.Notes and references
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418–2421 CrossRef CAS PubMed.
- J. X. Wang, T. T. Cai, J. L. Li, R. X. Zhuo and X. Z. Zhang, RSC Adv., 2014, 4, 14993 RSC.
- (a) Y. Jin, X. D. Xu, S. Chen, S. X. Cheng, X. Z. Zhang and R. X. Zhuo, Macromol. Rapid Commun., 2008, 29, 1726 CrossRef CAS; (b) A. J. V. Hell, A. Klymchenko, P. P. Burgers, E. E. Moret, W. Jiskoot, W. E. Hennink, D. J. A. Crommelin and E. Mastrobattista, J. Phys. Chem. B, 2010, 114, 11046 CrossRef PubMed; (c) E. Longo, M. Crisma, F. Formaggio, C. Toniolo and A. Moretto, Polym. Chem., 2013, 45, 516 CAS; (d) X. Zhao, F. Pan and J. R. Lu, Prog. Nat. Sci., 2008, 18, 653 CrossRef CAS; (e) D. Mandal, A. N. Shirazi and K. Parang, Org. Biomol. Chem., 2014, 12, 3544–3561 RSC; (f) G. Fuks, R. M. Tlom and F. Gauffre, Chem. Soc. Rev., 2011, 40, 2475 RSC; (g) Z. Luo and S. Zhang, Chem. Soc. Rev., 2012, 4736 RSC; (h) R. H. Zha, S. Sur, J. Boekhoven, H. Y. Shi, M. Zhang and S. I. Stupp, Acta Biomater., 2015, 12, 1 CrossRef CAS PubMed; (i) P. K. Koumkoua, C. Aisenbrey, E. Salnikov, O. Rifi and B. Bechinger, J. Pept. Sci., 2014, 20, 526 CrossRef PubMed; (j) Peptide materials from nanostructures to applications, ed. C. Aleman, A. Bianco and M. Venanzi, ISBN: 978-1-119-95373-9, Wiley-VCH, 2013 Search PubMed; (k) S. Zhang, D. M. Marini, W. Hwang and S. Santoso, Curr. Opin. Chem. Biol., 2002, 6, 865 CrossRef CAS PubMed; (l) A. Dehsorkhi, V. Castelletto and I. W. Hamley, J. Pept. Sci., 2014, 20, 453 CrossRef CAS PubMed; (m) P. Moitra, K. Kumar, P. Kondaiah and S. Bhattacharya, Angew. Chem., Int. Ed., 2014, 53, 1113 CrossRef CAS PubMed.
- (a) C. M. Rufo, Y. S. Moroz, O. V. Moroz, J. Stöhr, T. A. Smith, X. Hu, W. F. DeGrado and I. V. Korendovych, Nat. Chem., 2014, 6, 303 CrossRef CAS PubMed; (b) S. Marchesan, A. V. Vargiu and K. E. Styan, Molecules, 2015, 19775 CrossRef CAS PubMed; (c) C. H. Gçrbitz, Chem.–Eur. J., 2007, 1022 CrossRef PubMed; (d) A. Bonetti, S. Pellegrino, P. Das, S. Yuran, R. Bucci, N. Ferri, F. Meneghetti, C. Castellano, M. Reches and M. L. Gelmi, Org. Lett., 2015, 17, 4468–4471 CrossRef CAS PubMed.
- (a) J. J. Panda and V. S. Chauhan, Polym. Chem., 2014, 5, 4418–4436 RSC; (b) Y. Yang, U. Khoe, X. Wang, A. Horii, H. Yokoi and S. Zhang, Nano Today, 2009, 193 CAS.
- (a) L. Gentilucci, D. R. Marco and L. Cerisoli, Curr. Pharm. Des., 2010, 16, 3185–3203 CrossRef CAS PubMed; (b) S. Pellegrino, A. Contini, F. Clerici, A. Gori, D. Nava and M. L. Gelmi, Chem.–Eur. J., 2012, 18, 8705 CrossRef PubMed.
- (a) C. Toniolo, M. Crisma, F. Formaggio and C. Peggion, Biopolymers, 2001, 60, 396 CrossRef CAS PubMed and references therein; (b) P. Balaram, Curr. Opin. Struct. Biol., 1992, 2, 845 CrossRef CAS; (c) C. Toniolo, Janssen Chim. Acta, 1993, 11, 10 CAS; (d) P. Sudhanand, R. Balaji and P. Balaram, Biopolymers, 1995, 35, 11 CrossRef PubMed; (e) I. Torrini, M. Paglialunga Paradisi, G. Pagani Zecchini and G. Lucente, Synth. Commun., 1994, 24, 153 CrossRef CAS; (f) M. Cirilli, V. M. Coiro, A. Di Nola and F. Mazza, Biopolymers, 1998, 46, 239 CrossRef CAS; (g) C. Cativiela and M. D. Diaz-de-Villegas, Tetrahedron: Asymmetry, 1998, 9, 3517 CrossRef CAS; (h) R. Kaul, S. Banumathi, D. Velmurugan, R. Balaji Rao and P. Balaram, Biopolymers, 2000, 54, 159 CrossRef CAS PubMed; (i) C. Toniolo, Int. J. Pept. Protein Res., 1990, 35, 287 CrossRef CAS PubMed; (j) M. Savian, R. Iacovino, V. Menchise, E. Benedetti, G. M. Bonora, M. Gatos, L. Graci, F. Formaggio, M. Crisma and C. Toniolo, Biopolymers, 2000, 53, 200 CrossRef; (k) C. Cativiela and M. D. Diaz-de-Villegas, Tetrahedron: Asymmetry, 2000, 11, 645 CrossRef CAS; (l) A. Moretto, F. Formaggio, M. Crisma, C. Toniolo, M. Saviano, R. Iacovino, R. M. Vitale and E. Benedetti, J. Pept. Res., 2001, 57, 307 CrossRef CAS PubMed; (m) I. Maffucci, S. Pellegrino, J. Clayden and A. Contini, J. Phys. Chem. B, 2015, 119, 1350 CrossRef CAS PubMed.
- S. Pellegrino, A. Contini, M. L. Gelmi, L. Lo Presti, R. Soave and E. Erba, J. Org. Chem., 2014, 79, 3094 CrossRef CAS PubMed.
- (a) N. Zhou, X. Gao, Y. Lv, J. Cheng, W. Zhou and K. Liu, J. Pept. Sci., 2014, 20, 868 CrossRef CAS PubMed; (b) M. K. Chung, S. J. Lee, M. L. Waters and M. R. Gagné, J. Am. Chem. Soc., 2012, 134, 11430 CrossRef CAS PubMed; (c) D. Zanuy, G. Ballano, A. I. Jiménez, J. Casanovas, N. Haspel, C. Cativiela, D. Curcó, R. Nussinov and C. Alemán, J. Chem. Inf. Model., 2009, 49, 1623 CrossRef CAS PubMed.
- (a) A. Ruffoni, N. Ferri, S. K. Bernini, C. Ricci, A. Corsini, I. Maffuccci, F. Clerici and A. Contini, J. Med. Chem., 2014, 57, 2953 CrossRef CAS PubMed; (b) A. Ruffoni, A. Contini, R. Soave, L. Lo Presti, I. Esposto, I. Maffucci, D. Nava, S. Pellegrino, M. L. Gelmi and F. Clerici, RSC Adv., 2015, 5, 32643 RSC.
- (a) H. Imai, K. Kaira, N. Oriuchi, K. Shimizu, H. Tominaga, N. Yanagitani, N. Sunaga, T. Ishizuka, S. Nagamori, K. Promchan, T. Nakajima, N. Yamamoto, M. Mori and Y. Kanai, Anticancer Res., 2010, 30, 4819 CAS; (b) C. S. Kim, S.-H. Cho, H. S. Chun, S.-Y. Lee, H. Endou, Y. Kanai and D. K. Kim, Biol. Pharm. Bull., 2008, 31, 1096 CrossRef CAS PubMed.
- (a) M. J. Gattner, M. Ehrilch and M. Vrabel, Chem. Commun., 2014, 50, 12568 RSC; (b) J. C. Jewett and C. R. Bertozzi, Chem. Soc. Rev., 2010, 39, 1272 RSC; (c) S. Datz, C. Argyo, M. Gattner, V. Weiss, K. Brunner, J. Bretzler, C. von Schirnding, A. A. Torrano, F. Spada, M. Vrabel, H. Engelke, C. Bräuchle, T. Carella and T. Bein, Nanoscale, 2016, 8, 8101 RSC.
- P. A. Wade, K. J. Murray Jr, S. Shah-Patela and P. J. Carroll, Tetrahedron Lett., 2002, 43, 2585 CrossRef CAS.
- A. Thaqi, A. McCluskey and J. L. Scott, Tetrahedron Lett., 2008, 49, 6962 CrossRef CAS.
- J. Lu, S. C. Owen and M. S. Shoichet, Macromolecules, 2011, 44, 6002 CrossRef CAS PubMed.
- E. J. Cho, H. Holback, K. C. Liu, S. A. Abouelmagd, J. Park and Y. Yeo, Mol. Pharm., 2013, 10, 2093 CrossRef CAS PubMed.
- (a) K. Wutricht, NMR of Protein and Nucleic Acids, Wiley, New York, 1986 Search PubMed; (b) G. L. Millhauser, C. J. Stenland, P. Hanson, K. A. Bolin and F. J. M. van de Ven, J. Mol. Biol., 1997, 267, 963 CrossRef CAS PubMed; (c) G. L. Millhauser, Biochemistry, 1995, 34, 3873 CrossRef CAS PubMed.
- (a) J. D. Augspurger, V. A. Bindra, H. A. Scheraga and A. Kuki, Biochemistry, 1995, 34, 2566 CrossRef CAS PubMed; (b) N. J. Baxter and M. P. Williamson, J. Biomol. NMR, 1997, 9, 359 CrossRef CAS PubMed; (c) G. M. Bonora, C. Mapelli, C. Toniolo, R. R. Wilkening and E. S. Stevens, Int. J. Biol. Macromol., 1984, 6, 179 CrossRef CAS; (d) E. K. S. Vijayakumar and P. Balaram, Biopolymers, 1983, 22, 2133 CrossRef CAS PubMed.
- (a) R. Banerjee, S. Chattopadhyay and G. Basu, Proteins: Struct., Funct., Bioinf., 2009, 76, 184 CrossRef CAS PubMed; (b) G. Merutka, H. J. Dyson and P. E. Wright, J. Biomol. NMR, 1995, 5, 14 CrossRef CAS PubMed.
- Peptide 1 ΔCONH2 (Δδ/ΔT) = 5.7 ppb K−1 in CD3OH, ΔCONH2 (Δδ/ΔT) = 2.0 ppb K−1 in H2O/D2O; peptide 2 ΔCONH2 (Δδ/ΔT) = 5.8 ppb K−1 in CD3OH, ΔCONH2 (Δδ/ΔT) = 3.5 ppb K−1 in H2O/D2O, see ESI: Tables TS3–TS6.†.
- G. Jung, H. Bruckner, R. Bosch, W. Winter, H. Schaal and J. Strhle, Liebigs Ann. Chem., 1983, 1096 CrossRef CAS.
- R. Oekonomopulos, G. Jung and D. Leibfritz, Tetrahedron, 1982, 38, 2157 CrossRef CAS.
- S. J. Pike, V. Diemer, J. Raftery, S. J. Webb and J. Clayden, Chem.–Eur. J., 2014, 20, 15981 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17116h |
| This journal is © The Royal Society of Chemistry 2016 |




