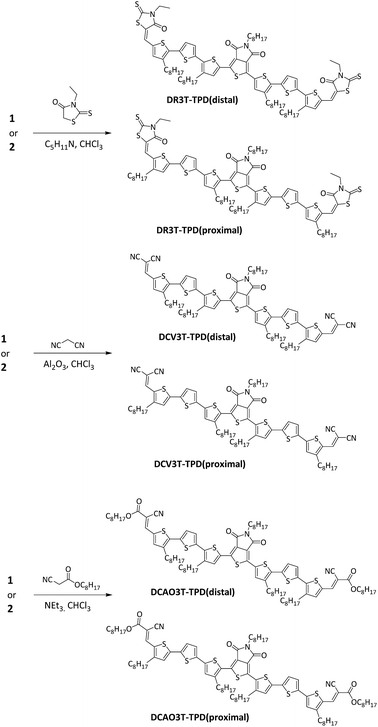
Effect of alkyl chain topology on the structure, optoelectronic properties and solar cell performance of thienopyrroledione-cored oligothiophene chromophores†
Bright Walker‡
a,
Seungjib Yum‡b,
Bomee Jangc,
Jihyeon Kimb,
Taehyo Kima,
Jin Young Kim*a and
Han Young Woo*c
aSchool of Energy and Chemical Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan 689-798, Korea. E-mail: jykim@unist.ac.kr
bDepartment of Cogno-Mechatronics Engineering, Pusan National University, Miryang 627-706, Korea
cDepartment of Chemistry, College of Science, Korea University, Seoul 02841, Korea. E-mail: hywoo@korea.ac.kr
First published on 9th August 2016
Abstract
We have investigated a series of oligothiophenes containing a central thienopyrroledione group with rhodanine, dicyanovinyl and octylcyanoacrylate end-capping groups. For each end capping group, two alkyl chain configurations were explored by appending n-octyl chains to the oligothiophenes in both proximal and distal topologies. Substitution of different alkyl topologies and end-capping groups altered not only intramolecular conformations but also intermolecular interactions, thus affecting frontier molecular orbitals and bulk properties such as optical, thermal transitions, solid state packing, and device properties. The electronic properties of the materials were probed in field effect transistors (FETs), single carrier diodes and bulk heterojunction (BHJ) solar cell devices. FET devices revealed that all materials behaved as p-type semiconductors with mobilities in the range of 10−5 to 10−3 cm2 V−1 s−1. In solar cell devices, we observed that the optimal end capping group was rhodanine, while the optimal alkyl chain configuration was the proximal configuration. The rhodanine capped molecule with proximal alkyl chain isomerism led to a power conversion efficiency of ∼2%. Grazing-incidence wide-angle X-ray scattering studies of this series of molecules revealed a strong tendency to undergo edge-on packing with no π–π stacking in the vertical direction, which may limit their performance in BHJ solar cells.
Introduction
Organic bulk heterojunction (BHJ) solar cells show great promise as solution-processable, low-cost, renewable and flexible energy sources.1–3 The potential advantages of organic BHJ solar cells have spurred an intense research effort which has resulted in rapid increases in power conversion efficiencies (PCEs) over ∼11%.4–6 Although most BHJ solar cells comprise polymeric donors, molecular p-type materials may also be used with an n-type, molecular fullerene derivative,7–12 termed “small molecule” BHJs, since both active layer components are non-polymeric molecular solids. These molecular materials offer advantages over their polymeric counterparts in terms of simplified synthesis and purification as well as improved batch-to-batch consistency as discrete molecules do not suffer from molecular weight variability or polydispersity issues as polymers do. Although polymer BHJs have yielded higher PCEs than small molecule BHJs to date, the highest efficiency small molecules devices (8–10%) reported recently are not far behind their polymeric counterparts.13–18 Among the most successful small molecule BHJ designs include oligothiophenes with terminal electron withdrawing groups (EWGs). A molecular design comprising heptathiophene with terminal EWGs was reported in 2010 by Chen and coworkers19 and found to yield relatively good performance at that time. Since then, improvements in molecular design such as incorporation of rhodanine end-capping groups and a benzodithiophene core have allowed control over electronic properties and resulted in substantial improvements in device efficiency.14–17,20Thienopyrroledione (TPD) is an electron withdrawing group that has been used successfully in polymer BHJ solar cells and recently in small molecule solar cells as well;21–25 yielding devices with relatively high open-circuit voltage (VOC). Additionally, alkyl chain topology is a critical factor which has to be considered when designing π-conjugated molecules.26–29 Careful consideration has to be taken to satisfy both solubility and efficient π–π interactions. For instance, Wu et al. demonstrated the importance of alkyl chain orientation in a dicyanomethylene-substituted 2,5-di(thiophen-2-yl)thieno-[3,2-b]thieno quinoid compound. Differences in alkyl chain substitution position led to significant changes in solid state molecular packing and significantly influenced charge transport behavior.28 Similarly, Wessendorf et al. demonstrated that alkyl chain orientation strongly affected material solubility and device performance.29
In this contribution, we report a series of oligothiophenes incorporating a central TPD group and terminal EWGs (rhodanine, dicyanovinyl and octylcyanoacrylate), as shown in Fig. 1. Their structural, thermal, optical, electrochemical and morphological properties were characterized for opto-electronic device applications. Further, we have examined the effect of proximal and distal alkyl chain topology on material properties. Alkyl chains were systematically appended on oligothiophene in proximal or distal position with respect to TPD core. Structural modification through EWGs and alkyl chain topology using the same oligothiophene based π-backbone led to significant differences in electronic structure, thermal behavior and solid state packing, thus affecting charge transport and solar cell device properties.
Experimental
Materials and syntheses
All chemical reagents were purchased from Aldrich, Tokyo Chemical Industry and Junsei Chemical and used without further purification. 5-Bromo-5′′-formyl-3,3′′-dioctyl-2,2′:5′,2′′-terthiophene,30,31 and 5-octylthieno[3,4-c]pyrrole-4,6-dione32 were synthesized according to previously published procedures. 5-Bromo-5′′-formyl-4,4′′-dioctyl-2,2′:5′,2′′-terthiophene was prepared by modification of previously reported procedures.30,31,33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give DCV3T-TPD. Yield: 300 mg (55%). 1H NMR (300 MHz, CDCl3, ppm): δ 7.80 (s, 2H), 7.36 (d, J = 3.9 Hz, 2H), 7.23 (d, J = 3.9 Hz, 2H), 7.15 (s, 2H), 7.12 (s, 2H), 3.65 (t, J = 7.2 Hz, 2H), 2.82 (t, J = 7.5 Hz, 4H), 2.74 (t, J = 7.5 Hz, 4H), 1.66 (m, 10H), 1.35 (m, 50H), 0.87 (m, 15H). 13C NMR (75 MHz, CDCl3, ppm): δ 162.12, 156.96, 147.26, 146.96, 145.66, 139.39, 138.02, 135.96, 134.76, 130.91, 129.02, 128,14, 127.34, 125.96, 125.78, 125.49, 114.89, 113.75, 31.87, 31.80, 31.18, 30.36, 30.08, 29.70, 29.58, 29.39, 29.37, 29.28, 29.23, 29.12, 28.52, 27.00, 22.63, 22.60, 13.99. MALDI-TOF: m/z = 1357.5.
1) to give DCV3T-TPD. Yield: 300 mg (55%). 1H NMR (300 MHz, CDCl3, ppm): δ 7.80 (s, 2H), 7.36 (d, J = 3.9 Hz, 2H), 7.23 (d, J = 3.9 Hz, 2H), 7.15 (s, 2H), 7.12 (s, 2H), 3.65 (t, J = 7.2 Hz, 2H), 2.82 (t, J = 7.5 Hz, 4H), 2.74 (t, J = 7.5 Hz, 4H), 1.66 (m, 10H), 1.35 (m, 50H), 0.87 (m, 15H). 13C NMR (75 MHz, CDCl3, ppm): δ 162.12, 156.96, 147.26, 146.96, 145.66, 139.39, 138.02, 135.96, 134.76, 130.91, 129.02, 128,14, 127.34, 125.96, 125.78, 125.49, 114.89, 113.75, 31.87, 31.80, 31.18, 30.36, 30.08, 29.70, 29.58, 29.39, 29.37, 29.28, 29.23, 29.12, 28.52, 27.00, 22.63, 22.60, 13.99. MALDI-TOF: m/z = 1357.5.Measurements
1H and 13C NMR spectra were recorded using a JEOL (JNM-AL300) FT NMR system operating at 300 and 75 MHz, respectively. Coupling reactions were carried out using a microwave reactor (Biotage Initiator). Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements were performed using a TA Instruments TGA 2050 thermogravimetric analyzer and DSC Q200 under a nitrogen atmosphere at a heating and cooling rate of 10 °C min−1. UV-vis absorption spectra were measured using a Jasco (V-630) spectrophotometer. Cyclic voltammetry (CV) was measured on a Versa STAT3 (Princeton Applied Research) with a three-electrode cell employing a platinum wire as the counter electrode, a platinum electrode as the working electrode, and a Ag/Ag+ electrode as the reference electrode in 0.1 M tetrabutylammonium tetrafluoroborate (Bu4NBF4) in CH2Cl2 at a scan rate of 50 mV s−1. The sample concentration was ∼1 mg mL−1 in CH2Cl2. All electrochemical solutions were purged with dry N2 for 15 min to deoxygenate the system prior to measurement. All measurements were calibrated against an internal standard of ferrocene (Fc), the ionization potential (IP) value of which was assumed to be −4.8 V for the Fc/Fc+ redox system.34 Grazing incidence wide angle X-ray scattering (GIWAXS) measurements were carried out at the PLS-II 9A U-SAXS beam line of Pohang Accelerator Laboratory, Korea.Solubility measurement
Solubility of each compound was determined following a reported procedure.35 A saturated solution of each compound was prepared by adding excess of each compound to a small amount of chloroform (∼50 mg mL−1). The slurry was stirred for 48 hours at room temperature then allowed to stand still for 12 hours. The slurry was then filtered through a 0.45 μm PTFE filter. The filtrate is assumed to be a saturated solution in chloroform. A 50 μL aliquot was then diluted to 10 mL with chloroform. The UV-vis absorption spectrum was acquired and the concentration determined using a standard calibration curve. The calibration curve was prepared by measuring the absorbance of 5 solutions of a given compound in chloroform with known concentrations. Extinction coefficient measurement was conducted using Beer–Lambert law. Absorption of diluted small molecule solution (concentration known) was measured using Jasco (V-630) spectrophotometer. Each experiment was repeated to ensure validity of the collected data.Solar cell device fabrication
Solar cell devices were fabricated on glass/ITO substrates which were cleaned with detergent, then ultra-sonicated in isopropyl alcohol and subsequently dried in an oven at 100 °C. A PEDOT:PSS layer (Baytron Clevios AI4083) was next deposited by spin casting at 5000 rpm for 40 s after filtration through a 0.45 μm cellulose acetate syringe filter, and baked at 140 °C for 15 min in air and transferred into a nitrogen filled glove box. The blended mixtures (∼17 mg mL−1 in CHCl3) of each donor material and phenyl-C71-butyric acid methyl ester (PC71BM) were deposited on top of the PEDOT:PSS layer yielding active layers 80 to 100 nm thick. Finally, 80 nm thick Al electrodes were thermally evaporated under vacuum (∼10−6 Torr). The deposited Al electrode defines the active area of the devices as 13 mm2. J–V measurements were collected with a Keithley 2635A source measurement unit under a nitrogen atmosphere, using simulated (AM1.5 G, 100 mW cm−2) solar radiation from a xenon arc lamp equipped with a high quality optical fiber to guide light. Eight solar cell devices were fabricated for each condition and the reported device characteristics represent the highest value for each condition while the average value is also reported in parentheses. External quantum efficiency (EQE) measurements were conducted in ambient air using an EQE system (Model QEX7) by PV measurements Inc. (Boulder, Colorado). Spectral mismatch factors between currents produced under xenon arc lamp and the standard AM1.5 G spectrum were found to be ∼10% or less.Field effect transistor (FET) fabrication
FET devices were fabricated on pre-cleaned silicon substrates with a 250 nm thermally grown SiO2 dielectric layer. Substrates were dried in an oven at 100 °C prior to device fabrication. Active layers were deposited by spin-coating a solution of each semiconductor (∼10 mg mL−1 in CHCl3) at 1500 rpm. Source and drain electrodes (Ag or Au) were deposited by thermal evaporation through a shadow mask. FET devices were tested under nitrogen using probe station and a Keithley 4200 semiconductor characterization system.Results and discussion
Synthesis and thermal properties
A series of oligothiophenes incorporating a central TPD group and terminal EWGs (rhodanine, dicyanovinyl and octylcyanoacrylate) were designed and synthesized by modulating proximal and distal alkyl chain topology on the molecular backbone. The synthetic routes of both proximal and distal formyl terminated precursors and the final small molecules of DR3T-TPD, DCV3T-TPD and DCAO3T-TPD are presented in Schemes 1 and 2. Direct heteroarylation coupling between bromoterthiophene and thienopyrrolodione in the presence of trans-di(μ-acetato)bis[o-(di-o-tolyl-phosphino)benzyl]dipalladium(II) and tris(o-methoxyphenyl)phosphine as a catalyst led to 5′,5′′-bis(formyl-4,4′′-dioctyl-2,2′:5′,2′′-terthiophene)-3,3′-5-octyl-thieno[3,4-c]pyrrole-4,6-dione and 5′,5′′-bis(formyl-3,3′′-dioctyl-2,2′:5′,2′′-terthiophene)-3,3′-5-octyl-thieno[3,4-c]pyrrole-4,6-dione. DR3T-TPD(proximal) and DR3T-TPD(distal) were prepared in 81% and 94% yield by coupling of aromatic aldehyde of compound 1 or compound 2 and 3-ethylrhodanine based on the modified Perkin reaction.36 The Knoevenagel condensation reaction was applied to the synthesis of DCV3T-TPD and DCAO3T-TPD using malononitrile or cyanoacetate. DCV3T-TPD(proximal), DCV3T-TPD(distal), DCAO3T-TPD(proximal), and DCAO3T-TPD(distal) were obtained in 55%, 37%, 93%, and 77% yield, respectively.Thermal transitions of the small molecules were investigated using TGA (Fig. S1†) and DSC with third heating and cooling loop of each compound to determine thermal transition temperatures (Fig. 2). Table 1 summarizes the thermal properties and solubility of the molecules. Changing the anchoring position of alkyl chains on the conjugated backbone led to substantial differences in thermal behavior. DR3T-TPD(distal) showed higher melting and crystallization temperatures by 53 and 34 °C, respectively, compared to DR3T-TPD(proximal), which indicates stronger intermolecular interactions for DR3T-TPD(distal) in solid state. We suspect that alkyl chain repulsion near the TPD core may disrupt the effective planarization of the π-backbone in the DR3T-TPD(proximal). The solubilities of DR3T-TPD(proximal) and DR3T-TPD(distal) in chloroform were determined to be ∼39 and ∼20 mg mL−1, respectively. Interestingly, however, as the rhodanine end-capping group was changed to dicyanovinyl group, the thermal transition trend was reversed. DCV3T-TPD(proximal) showed higher melting and crystallization temperatures by 29 and 26 °C compared to DCV3T-TPD(distal) which was consistent with the solubilities of DCV3T-TPD(proximal) and DCV3T-TPD(distal), which were determined to be ∼16 mg mL−1 and ∼21 mg mL−1 in chloroform. The proximal and distal topology and thermal behaviors did not show a simple correlation and the complicated interplay of several factors is expected including the end-capping EWG molecular structures. Substitution with the cyanoacrylate end-group resulted in similar thermal transitions with good solubility for both distal and proximal isomers. The more alkyl chains (seven vs. five octyl groups) on the DCAO3T-TPD backbone provides good solubility, decreases the phase transition temperatures and seems to wash out other competing interactions.
| Compound | Td (°C) | Tm (°C) | Tc (°C) | Solubility in CHCl3 (mg mL−1) |
|---|---|---|---|---|
| DR3T-TPD(distal) | 408 | 223.5 | 168.0 | ∼20 |
| DR3T-TPD(proximal) | 403 | 170.4 | 133.6 | ∼39 |
| DCV3T-TPD(distal) | 415 | 213.4 | 201.3 | ∼21 |
| DCV3T-TPD(proximal) | 400 | 242.4 | 227.0 | ∼16 |
| DCAO3T-TPD(distal) | 362 | 155.6 | 137.0 | >50 |
| DCAO3T-TPD(proximal) | 371 | 155.1 | 113.4 | >50 |
Fig. 3 shows the optical absorption spectra of the oligothiophene molecules in chloroform and in film. All compounds in solution exhibit a low energy absorption band (λmax ∼500 nm) and weaker high energy absorption band (λmax ∼300 nm) without defined structures, indicating negligible intermolecular aggregation in solution. The absorption spectra show slight difference in spectral shape, λmax and intensity depending on the alkyl chain topology and end-capping groups. Proximal compounds exhibited blue-shifted absorption onsets compared to distal compounds in both solution and film. This blue-shift can be attributed to twisting and reduced conjugation between the TPD core and inner thiophene group caused by the inner alkyl chain; a similar effect was observed by Wessendorf et al., where dithienopyrrole cored oligothiophenes exhibited blue-shifted absorption in solution when alkyl chains were oriented towards the core of the molecule.29 These optical data are summarized in Table 2. For all of the molecules, thin film spectra were red-shifted by ∼70 nm and showed a shoulder peak at ∼650 nm, which can be attributed to planarization of the molecule and intermolecular π–π interactions. Especially, DCV3T-TPD(proximal) showed a pronounced shoulder peak in film, suggesting tighter π–π stacking compared to the distal isomer, which shows a very good agreement with the DSC measurements. Different end-capping groups and distal/proximal topology interplayed together and affected the degree of intermolecular π–π interactions of the compounds.
 | ||
| Fig. 3 UV-Vis absorption spectra of (a) DR3T-TPD, (b) DCV3T-TPD and (c) DCAO3T-TPD in chloroform (dashed lines) and in film (solid lines). | ||
| Compound | λmax, sol (nm) | εmax solution (M−1 cm−1) | λmax, film (nm) | Eoptga (eV) | HOMO (eV) | LUMO (eV) | Ecvg (eV) |
|---|---|---|---|---|---|---|---|
| a Optical band gaps were estimated from the onset wavelength in film absorption spectra. | |||||||
| DR3T-TPD(distal) | 510 | 1.13 × 105 | 589 | 1.71 | −5.00 | −3.46 | 1.54 |
| DR3T-TPD(proximal) | 522 | 1.13 × 105 | 571 | 1.78 | −5.16 | −3.46 | 1.70 |
| DCV3T-TPD(distal) | 510 | 6.58 × 104 | 573 | 1.73 | −5.28 | −3.50 | 1.78 |
| DCV3T-TPD(proximal) | 514 | 7.39 × 104 | 630 | 1.79 | −5.36 | −3.49 | 1.87 |
| DCAO3T-TPD (distal) | 494 | 5.17 × 104 | 554 | 1.80 | −5.24 | −3.44 | 1.80 |
| DCAO3T-TPD(proximal) | 498 | 6.78 × 104 | 551 | 1.86 | −5.32 | −3.43 | 1.89 |
Electrochemical properties were measured in solution using CV. These data are shown in Fig. S2† and summarized in Table 2. Regardless of the end-capping group, distal compounds showed higher highest occupied molecular orbital (HOMO) levels than the proximal compounds (by ca. 0.08–0.16 eV), while the lowest unoccupied molecular orbitals (LUMOs) remained unaffected. Alkyl chains near TPD in the proximal compounds may disturb the effective overlap of the π-orbitals between the electron rich thiophene units and electron deficient TPD and end-capping group units, leading to less effective conjugation compared to distal compounds. Also, we observed that HOMO and LUMO levels could be controlled via incorporation of different end-capping groups.37 Although acceptor groups are expected to primarily affect LUMO orbitals, we observed that changes to the terminal acceptor moiety influenced both HOMO and LUMO values, which has been previously observed.38 Differences in HOMO energies may be attributed to different effects that terminal groups have on the solid state packing as well as inductive affects due to the delocalized HOMO orbital spatially overlapping the terminal electron withdrawing group. Dicyanovinyl substituted compounds exhibited the deepest HOMO and LUMO levels, reflecting the strong electron withdrawing effect of the two nitrile moieties in the dicyanovinyl group, while rhodanine substitution yielded the narrowest band gaps, with deeper LUMO levels than octylcyanoacrylate and the highest HOMO energy bands, and octylcyanoacrylate moieties yielded the highest LUMO energies and widest band gaps. These trends are consistent with observations made by Luponosov et al., where it was found that dicyanovinyl groups caused a stronger deepening of LUMO bands than rhodanine groups, yet rhodanine groups yielded narrower band gaps in the film state than dicyanovinyl groups.20 These thermal, optical and electrochemical properties demonstrate that systematic tuning through variation alkyl anchoring positions and end-capping substituents not only modulates optical properties and energy bands, but also has a noticeable impact on bulk properties such as molecular packing and solubility.
Photovoltaic properties
BHJ solar cell devices were prepared from mixtures of the donor materials with PC71BM using the architecture ITO/PEDOT:PSS/donor:PC71BM/Al. For each material, a range of donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor ratios (by weight) were explored including 3
acceptor ratios (by weight) were explored including 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 4
7, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 5
6, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 6
5, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 7
4 and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, as well as thermal annealing at several temperatures (80, 110, and 140 °C). Optimized J–V curves and EQE spectra are shown in Fig. 4, while optimized device parameters are summarized in Table 3. Detailed J–V characteristics and EQE of as-cast devices using different materials and blend ratios are reported in Fig. S3 and S4,† respectively and a complete table of device parameters can be found in Table S1.†
3, as well as thermal annealing at several temperatures (80, 110, and 140 °C). Optimized J–V curves and EQE spectra are shown in Fig. 4, while optimized device parameters are summarized in Table 3. Detailed J–V characteristics and EQE of as-cast devices using different materials and blend ratios are reported in Fig. S3 and S4,† respectively and a complete table of device parameters can be found in Table S1.†
| Material | Isomer | Blend ratio (D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A) A) |
JSC (mA cm−2) | VOC (V) | FF | PCE (%) |
|---|---|---|---|---|---|---|
| DR3T-TPD | Proximal | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
4.89 | 0.96 | 0.43 | 1.98 (1.77) |
| DR3T-TPD | Distal | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
2.40 | 0.93 | 0.32 | 0.71 (0.67) |
| DCV3T-TPD | Proximal | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
3.18 | 0.79 | 0.51 | 1.28 (1.21) |
| DCV3T-TPD | Distal | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
1.10 | 0.77 | 0.29 | 0.25 (0.23) |
| DCAO3T-TPD | Proximal | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1.01 | 0.89 | 0.39 | 0.35 (0.31) |
| DCAO3T-TPD | Distal | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0.66 | 0.77 | 0.39 | 0.20 (0.17) |
Optimal PCEs of 1.98, 1.28 and 0.35% were measured for the proximal isomers of DR3T-TPD, DCV3T-TPD and DCAO3T-TPD, respectively, while 0.71, 0.25 and 0.20% were the optimal PCEs observed for the distal isomers of DR3T-TPD, DCV3T-TPD and DCAO3T-TPD. In all cases, the proximal isomer performed significantly better than the distal isomer. Among the different end-groups, the rhodanine-containing DR3T-TPD(proximal) showed the best overall performance. The greatest short-circuit current density (JSC) was produced by DR3T-TPD(proximal):PC71BM in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 blend ratio (4.89 mA cm−2), while DR3T-TPD(distal):PC71BM yielded a maximum JSC of 2.40 mA cm−2 with the 3
6 blend ratio (4.89 mA cm−2), while DR3T-TPD(distal):PC71BM yielded a maximum JSC of 2.40 mA cm−2 with the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 blend ratio. For comparison, integrating the EQE spectra of these devices yielded calculated JSCs of 4.84 and 2.21 mA cm−2, respectively. Optimal ratios for both DCV3T-TPD proximal and distal isomers were found to be 3
7 blend ratio. For comparison, integrating the EQE spectra of these devices yielded calculated JSCs of 4.84 and 2.21 mA cm−2, respectively. Optimal ratios for both DCV3T-TPD proximal and distal isomers were found to be 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and yielded somewhat lower JSCs of 3.18 and 1.10 mA cm−2, respectively. Optimal ratios for DCAO3T-TPD(proximal) and DCAO3T-TPD(distal) were 6
7 and yielded somewhat lower JSCs of 3.18 and 1.10 mA cm−2, respectively. Optimal ratios for DCAO3T-TPD(proximal) and DCAO3T-TPD(distal) were 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in both cases and yielded JSCs of 1.01 and 0.66 mA cm−2, respectively. Although the HOMO bands of both DR3T-TPD(proximal) and DR3T-TPD(distal) (−5.16 and −5.00 eV respectively) were measured to be higher than the other structures by CV (−5.36, −5.28, −5.32 and −5.24 eV for DCV3T-TPD(proximal), DCV3T-TPD(distal), DCAO3T-TPD(proximal) and DCAO3T-TPD(distal), respectively), the rhodanine-capped DR3T-TPD molecules exhibited the largest VOC of 0.93–0.96 V. DCV3T-TPD(proximal), DCV3T-TPD(distal), DCAO3T-TPD(proximal) and DCAO3T-TPD(distal) yielded VOCs of 0.79, 0.77, 0.89 and 0.77 V, respectively. Although trends in the observed VOC did not correlate exactly to trends observed in the HOMO bands of materials with different end-groups, it is noteworthy that the proximal isomers produced the greater VOC than the distal isomers in all cases, consistent with the CV results. Examining the EQE spectra indicates that all materials exhibit similar photocurrent onsets near ∼700 nm and showed good consistency with the measured UV-vis characteristics.
4 in both cases and yielded JSCs of 1.01 and 0.66 mA cm−2, respectively. Although the HOMO bands of both DR3T-TPD(proximal) and DR3T-TPD(distal) (−5.16 and −5.00 eV respectively) were measured to be higher than the other structures by CV (−5.36, −5.28, −5.32 and −5.24 eV for DCV3T-TPD(proximal), DCV3T-TPD(distal), DCAO3T-TPD(proximal) and DCAO3T-TPD(distal), respectively), the rhodanine-capped DR3T-TPD molecules exhibited the largest VOC of 0.93–0.96 V. DCV3T-TPD(proximal), DCV3T-TPD(distal), DCAO3T-TPD(proximal) and DCAO3T-TPD(distal) yielded VOCs of 0.79, 0.77, 0.89 and 0.77 V, respectively. Although trends in the observed VOC did not correlate exactly to trends observed in the HOMO bands of materials with different end-groups, it is noteworthy that the proximal isomers produced the greater VOC than the distal isomers in all cases, consistent with the CV results. Examining the EQE spectra indicates that all materials exhibit similar photocurrent onsets near ∼700 nm and showed good consistency with the measured UV-vis characteristics.
Given the desirable device properties of DR3T-TPD(proximal), this material was selected for further detailed optimization. Thermal annealing consistently led to a decrease in device performance due to decreases in JSC and fill factor (FF). However, gentle thermal annealing at 80 °C was found to lead to an increase in VOC. For the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio annealed at 80 °C, a maximum VOC of 1.01 V was observed (Table S2†). Solvent additives including 1-chloronaphthalene (CN), 1,8-diiodooctane (DIO), diphenylether (DPE) and polydimethylsiloxane (PDMS) were also explored in the processing of DR3T-TPD(proximal); the photovoltaic parameters are listed in Table S3.† An additive concentration was adjusted around ∼0.6% as this is within the range of additive concentrations which work well with small molecule BHJs.39–41 However, little positive effects were measured with these series of processing additives. Only a slight increase in the PCE (2.01%) was achieved by the addition of 0.06% PDMS.13,42
5 ratio annealed at 80 °C, a maximum VOC of 1.01 V was observed (Table S2†). Solvent additives including 1-chloronaphthalene (CN), 1,8-diiodooctane (DIO), diphenylether (DPE) and polydimethylsiloxane (PDMS) were also explored in the processing of DR3T-TPD(proximal); the photovoltaic parameters are listed in Table S3.† An additive concentration was adjusted around ∼0.6% as this is within the range of additive concentrations which work well with small molecule BHJs.39–41 However, little positive effects were measured with these series of processing additives. Only a slight increase in the PCE (2.01%) was achieved by the addition of 0.06% PDMS.13,42
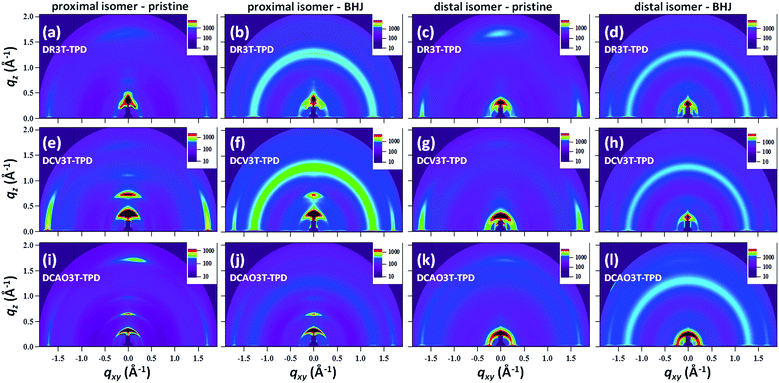
Grazing incidence wide angle X-ray scattering BHJ morphology
In order to probe the solid state packing structure and ordering in the pristine and BHJ blend films, GIWAXS measurements were performed. Two dimensional GIWAXS plots for pristine films of each material as well as optimal BHJ blends with PC71BM are shown in Fig. 5. The corresponding horizontal and vertical line cuts are included in the ESI (Fig. S5†). In the GIWAXS patterns, the distal isomers exhibit fewer and weaker peaks compared to the proximal isomers. Additionally, it is apparent that all of the BHJ blend films (2nd and 4th columns) show π–π stacking peaks (q > 1.5 Å−1) in the xy direction, but completely lack π–π stacking in the z direction.Pristine DR3T-TPD(proximal) films exhibited both lamellar (100) and π–π stacking (010) diffraction peaks at ∼1.72 Å−1 in both in-plane and out of plane directions. A weaker (200) lamellar peak is also observed in the out of plane direction in the pristine film. In the blend film, the (100) lamellar peak is much stronger in the out-of-plane direction while the π–π stacking (010) peak is present only in the in-plane direction, indicating that blending with PC71BM caused the material to favor an edge-on packing orientation relative to the substrate. The isomer DR3T-TPD(distal) showed similar tendencies with weaker diffraction peaks than the proximal isomer. In the pristine DR3T-TPD(distal) films, lamellar (100) and π–π stacking (010) peaks were both apparent. Upon blending with PC71BM, the π–π stacking peak disappeared in the out-of-plane direction, while the strong lamellar peak remained, indicating that an edge-on packing orientation was preferentially adopted by the distal isomer as well as the proximal. Compared to the rhodanine-capped molecule, DCV3T-TPD(proximal) showed an even stronger tendency towards edge-on packing. For the pristine films, only weak in-plane lamellar peaks (100) and (200) were apparent while the π–π stacking peak (∼1.74 Å−1) was much more intense in the xy direction. The very strong (010) peak in the in-plane direction is interpreted to be the origin of the strong shoulder peak in the UV-vis spectrum (see Fig. 3b). In contrast, the out of plane direction exhibited a series of sharp and strong lamellar peaks which can be assigned to (100), (200) and (300) diffraction planes with a very weak π–π stacking (010) peak. The BHJ films exhibited the same trend, with the appearance of a PC71BM peak at 1.33 Å−1. The DCV3T-TPD(distal) isomer showed the same trends as the proximal isomer, though with much weaker peaks and the absence of clear (200) or (300) diffractions in the out-of-plane direction. The octylcyanoacrylate capped molecules also showed very similar tendencies as the dicyanovinyl capped molecules, exhibiting a tendency towards edge-on packing in the BHJ films. In addition, the proximal isomers showed a tighter interlamellar packing with d-spacing of 16–19 Å (calculated from (100) peak in the z direction) in both pristine and BHJ films, compared to that (19–24 Å) of the distal isomers for all kinds of TPD-containing structures. In contrary, the π–π stacking distances (in the out-of-plane and/or in-plane direction) calculated for all of the pristine and blend films were found to be very similar at 3.66 ± 0.02 Å, 3.62 ± 0.02 Å and 3.62 ± 0.02 Å for DR3T-TPD, DCV3T-TPD and DCAO3T-TPD materials, and did not vary significantly for different alkyl chain topologies, in horizontal/vertical orientations or in pristine/BHJ films.
Charge carrier mobilities
FET devices were also prepared in order to probe the charge carrier mobility for each material. Transfer curves are reported in the ESI (Fig. S6†), while a summary of device parameters is included in Table 4. Silver electrodes were first used in order to facilitate injection of both electrons and holes, however all of the materials exhibited only p-channel operation. Hole mobilities (μh) were subsequently calculated using devices with Au electrodes to minimize contact resistance. Although the highest solar cell performance was observed in the rhodanine-capped molecules, the highest μh among the materials was observed in the DCV3T-TPD(proximal) material (9.0 × 10−4 cm2 V−1 s−1). The pronounced edge-on orientation of DCV3T-TPD(proximal) in GIWAXS shows a good agreement with FET mobility measurements. The distal isomer DCV3T-TPD(distal) showed somewhat lower μh of 2.7 × 10−5 cm2 V−1 s−1. Both rhodanine capped molecules exhibited similar μh of 1.7 and 1.8 × 10−4 cm2 V−1 s−1 for DR3T-TPD(proximal) and DR3T-TPD(distal), respectively, while the cyanoacrylate capped molecules exhibited the lowest mobilities (3.4 and 1.3 × 10−5 cm2 V−1 s−1 for DCAO3T-TPD(proximal) and DCAO3T-TPD(distal), respectively), presumably due to the presence of additional insulating octyl chains which are not present in the other molecules.| Material | Isomer | μh (cm2 V−1 s−1) | On/off | Vth (V) |
|---|---|---|---|---|
| DR3T-TPD | Proximal | 1.7 × 10−4 | 1.7 × 103 | −3.5 |
| DR3T-TPD | Distal | 1.8 × 10−4 | 2.6 × 103 | −12.5 |
| DCV3T-TPD | Proximal | 9.0 × 10−4 | 2.3 × 102 | −31.5 |
| DCV3T-TPD | Distal | 2.7 × 10−5 | 1.9 × 102 | −11.1 |
| DCAO3T-TPD | Proximal | 3.4 × 10−5 | 1.5 × 101 | −13.8 |
| DCAO3T-TPD | Distal | 1.3 × 10−5 | 1.3 × 103 | −21 |
In order to further understand the charge carrier transport in these materials, the space charge limited current (SCLC) technique was used to evaluate hole transport in the vertical direction.43,44 An architecture of ITO/PEDOT:PSS/active material/Au was used to ensure ohmic injection of holes at both electrodes and block electron injection, resulting in hole-only carrier transport. The J–V curves are shown in Fig. S7,† while the mobility values are summarized in Table S4.† First, the hole mobilities of the pristine films were measured. The highest hole mobility was observed for the rhodanine capped molecules, with the values of 2.5 × 10−4 and 7.2 × 10−5 cm2 V−1 s−1 for the proximal and distal isomers, respectively. This trend is consistent with the GIWAXS data, which show that the rhodanine capped molecules exhibited the most pronounced out of plane π–π stacking, which is conducive to vertical charge carrier transport. The dicyanovinyl and cyanoacrylate moieties resulted in successively lower values of 1.6 × 10−4, 1.8 × 10−5, 6.1 × 10−6 and 3.3 × 10−6 cm2 V−1 s−1 for DCV3T-TPD(proximal), DCV3T-TPD(distal), DCAO3T-TPD(proximal) and DCAO3T-TPD(distal), respectively. These values show that the vertical carrier mobilities are larger for the proximal isomers, consistent with the measured GIWAXS data.
For comparison, the hole mobilities of optimized BHJ blends were also measured. The mobilities were again found to be highest for the rhodanine end capped molecules (1.0 × 10−5 and 8.3 × 10−7 cm2 V−1 s−1 for DR3T-TPD(proximal):PC71BM and DR3T-TPD(distal):PC71BM, respectively), however were almost two orders of magnitude lower than the pristine films. Values for the dicyanovinyl and cyanoacrylate groups were on the order of 10−6 or 10−7 cm2 V−1 s−1 and again showed lower values for the distal isomers. These observations are consistent with GIWAXS and photovoltaic device characteristics. The blending with PC71BM may significantly disrupt π–π stacking in the vertical direction compared to the pristine materials.
Conclusions
A series of TPD containing small molecular donors were designed, synthesized, characterized and investigated in bulk heterojunction solar cells and FET devices. Alkyl chains were strategically appended on the oligothiophene π-backbone in proximal and distal configurations with respect to central TPD core. Structural manipulation through alkyl chain topology and end-capping group had significant effect on intermolecular interactions and solid state packing, as was revealed through thermal transitions, solubilities, CV measurements, UV absorption profiles and GIWAXS measurements. The materials exhibited suitable band gaps and energy band structures for use in solar cells including absorption onsets near 700 nm and HOMO levels in the range of 5.0–5.3 eV, leading to solar cells which generated photocurrent throughout the visible spectrum and exhibited VOC of up to ∼1 V. Among the end-capping groups, rhodanine was found to yield the best solar cell performance while the proximal alkyl chain configuration was found to be optimal. Optimized solar cells based on the proximal isomer of the rhodanine capped DR3T-TPD(proximal) with PC71BM showed a PCE of ∼2%. The strong tendency of this series of molecules to undergo edge-on packing leads to poor π–π stacking of BHJ films in the vertical direction, as revealed by GIWAXS data. This tendency is also confirmed by charge carrier mobility measurements, which show that vertical carrier transport is relatively low in these materials compared to horizontal transport. We conclude that the undesirable packing arrangement and inhibited charge transport to the electrodes in solar cell devices limit their performance. Different end-capping groups and distal/proximal topology interplayed together and affected the degree of intermolecular organization and the resulting device properties. This report marks the first detailed study on the effect of alkyl chain topology on the structural and optoelectronic properties of TPD-cored small molecules and may serve as a guide to aid in the alkyl chain design to further optimize small molecule photovoltaic chromophores.Acknowledgements
This work was supported by the National Research Foundation (NRF) of Korea (2015R1A2A1A15055605, 2015M1A2A2057506, 2015H1D3A1062473). This work was also supported by the New & Renewable Energy Core Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), Korea (No. 20133030011330).Notes and references
- T. Ameri, P. Khoram, J. Min and C. J. Brabec, Adv. Mater., 2013, 25, 4245–4266 CrossRef CAS PubMed.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789–794 CrossRef CAS.
- A. J. Heeger, Adv. Mater., 2014, 26, 10–28 CrossRef CAS PubMed.
- M. A. Green, K. Emery, Y. Hishikawa, W. Warta and E. D. Dunlop, Prog. Photovolt: Res. Appl., 2014, 22, 1–9 CrossRef.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- W. Zhao, D. Qian, S. Zhang, S. Li, O. Inganäs, F. Gao and J. Hou, Adv. Mater., 2016, 28, 4734–4739 CrossRef CAS PubMed.
- M. T. Lloyd, J. E. Anthony and G. G. Malliaras, Mater. Today, 2007, 10, 34–41 CrossRef CAS.
- J. Roncali, P. Leriche and P. Blanchard, Adv. Mater., 2014, 26, 3821–3838 CrossRef CAS PubMed.
- B. Walker, C. Kim and T.-Q. Nguyen, Chem. Mater., 2011, 23, 470–482 CrossRef CAS.
- A. Mishra and P. Bäuerle, Angew. Chem., Int. Ed., 2012, 51, 2020–2067 CrossRef CAS PubMed.
- J. E. Coughlin, Z. B. Henson, G. C. Welch and G. C. Bazan, Acc. Chem. Res., 2014, 47, 257–270 CrossRef CAS PubMed.
- K. Sun, Z. Xiao, S. Lu, W. Zajaczkowski, W. Pisula, E. Hanssen, J. M. White, R. M. Williamson, J. Subbiah, J. Ouyang, A. B. Holmes, W. W. H. Wong and D. J. Jones, Nat. Commun., 2015, 6, 6013 CrossRef CAS PubMed.
- A. K. K. Kyaw, D. H. Wang, D. Wynands, J. Zhang, T.-Q. Nguyen, G. C. Bazan and A. J. Heeger, Nano Lett., 2013, 13, 3796–3801 CrossRef CAS PubMed.
- J.-L. Wang, Q.-R. Yin, J.-S. Miao, Z. Wu, Z.-F. Chang, Y. Cao, R.-B. Zhang, J.-Y. Wang, H.-B. Wu and Y. Cao, Adv. Funct. Mater., 2015, 25, 3514–3523 CrossRef CAS.
- B. Kan, M. Li, Q. Zhang, F. Liu, X. Wan, Y. Wang, W. Ni, G. Long, X. Yang, H. Feng, Y. Zuo, M. Zhang, F. Huang, Y. Cao, T. P. Russell and Y. Chen, J. Am. Chem. Soc., 2015, 137, 3886–3893 CrossRef CAS PubMed.
- Y. Liu, C.-C. Chen, Z. Hong, J. Gao, Y. (Michael) Yang, H. Zhou, L. Dou, G. Li and Y. Yang, Sci. Rep., 2013, 3, 3356 Search PubMed.
- Q. Zhang, B. Kan, F. Liu, G. Long, X. Wan, X. Chen, Y. Zuo, W. Ni, H. Zhang, M. Li, Z. Hu, F. Huang, Y. Cao, Z. Liang, M. Zhang, T. P. Russell and Y. Chen, Nat. Photonics, 2015, 9, 35–41 CrossRef CAS.
- B. Kan, Q. Zhang, M. Li, X. Wan, W. Ni, G. Long, Y. Wang, X. Yang, H. Feng and Y. Chen, J. Am. Chem. Soc., 2014, 136, 15529–15532 CrossRef CAS PubMed.
- B. Yin, L. Yang, Y. Liu, Y. Chen, Q. Qi, F. Zhang and S. Yin, Appl. Phys. Lett., 2010, 97, 23303 CrossRef.
- Y. N. Luponosov, J. Min, A. N. Solodukhin, O. V. Kozlov, M. A. Obrezkova, S. M. Peregudova, T. Ameri, S. N. Chvalun, M. S. Pshenichnikov, C. J. Brabec and S. A. Ponomarenko, Org. Electron., 2016, 32, 157–168 CrossRef CAS.
- Y. Zhang, S. K. Hau, H.-L. Yip, Y. Sun, O. Acton and A. K.-Y. Jen, Chem. Mater., 2010, 22, 2696–2698 CrossRef CAS.
- J. A. Bartelt, Z. M. Beiley, E. T. Hoke, W. R. Mateker, J. D. Douglas, B. A. Collins, J. R. Tumbleston, K. R. Graham, A. Amassian, H. Ade, J. M. J. Fréchet, M. F. Toney and M. D. McGehee, Adv. Energy Mater., 2013, 3, 364–374 CrossRef CAS.
- J. Yuan, Z. Zhai, H. Dong, J. Li, Z. Jiang, Y. Li and W. Ma, Adv. Funct. Mater., 2012, 23, 885–892 CrossRef.
- H.-Y. Wang, J. Gao, L.-J. Gu, J.-H. Wan, W. Wei and F. Liu, J. Mater. Chem. A, 2013, 1, 5875–5885 CAS.
- J. Kim, C. Eun Song, S. K. Lee and E. Lim, Bull. Korean Chem. Soc., 2016, 37, 161–165 CrossRef CAS.
- J. Kumagai, K. Hirano, T. Satoh, S. Seki and M. Miura, J. Phys. Chem. B, 2011, 115, 8446–8452 CrossRef CAS PubMed.
- J. Liu, R. Zhang, G. Sauvé, T. Kowalewski and R. D. McCullough, J. Am. Chem. Soc., 2008, 130, 13167–13176 CrossRef CAS PubMed.
- Q. Wu, S. Ren, M. Wang, X. Qiao, H. Li, X. Gao, X. Yang and D. Zhu, Adv. Funct. Mater., 2013, 23, 2277–2284 CrossRef CAS.
- C. D. Wessendorf, G. L. Schulz, A. Mishra, P. Kar, I. Ata, M. Weidelener, M. Urdanpilleta, J. Hanisch, E. Mena-Osteritz and M. Lindén, Adv. Energy Mater., 2014, 4, 1400266 CrossRef.
- C. Saravanan, C.-L. Liu, Y.-M. Chang, J.-D. Lu, Y.-J. Hsieh, S.-P. Rwei and L. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 6133–6141 CAS.
- J. Zhou, X. Wan, Y. Liu, G. Long, F. Wang, Z. Li, Y. Zuo, C. Li and Y. Chen, Chem. Mater., 2011, 23, 4666–4668 CrossRef CAS.
- C. B. Nielsen and T. Bjørnholm, Org. Lett., 2004, 6, 3381–3384 CrossRef CAS PubMed.
- R. S. Loewe, P. C. Ewbank, J. Liu, L. Zhai and R. D. McCullough, Macromolecules, 2001, 34, 4324–4333 CrossRef CAS.
- Y. Sun, G. C. Welch, W. L. Leong, C. J. Takacs, G. C. Bazan and A. J. Heeger, Nat. Mater., 2012, 11, 44–48 CrossRef CAS PubMed.
- Z. B. Henson, G. C. Welch, T. van der Poll and G. C. Bazan, J. Am. Chem. Soc., 2012, 134, 3766–3779 CrossRef CAS PubMed.
- D. W. Mayo, R. M. Pike and D. C. Forbes, Microscale Organic Laboratory : with Multistep And Multiscale Syntheses, J. Wiley & Sons, Hoboken, NJ, 5th Ed, 2011, p. 291 Search PubMed.
- G. Long, X. Wan, B. Kan, Y. Liu, G. He, Z. Li, Y. Zhang, Y. Zhang, Q. Zhang, M. Zhang and Y. Chen, Adv. Energy Mater., 2013, 3, 639–646 CrossRef CAS.
- M. E. Kose, J. Phys. Chem. A, 2012, 116, 12503–12509 CrossRef CAS PubMed.
- H. Fan, H. Shang, Y. Li and X. Zhan, Appl. Phys. Lett., 2010, 97, 133302 CrossRef.
- B. Walker, J. Liu, C. Kim, G. C. Welch, J. K. Park, J. Lin, P. Zalar, C. M. Proctor, J. H. Seo, G. C. Bazan and T.-Q. Nguyen, Energy Environ. Sci., 2013, 6, 952–962 CAS.
- T. S. van der Poll, J. A. Love, T.-Q. Nguyen and G. C. Bazan, Adv. Mater., 2012, 24, 3646–3649 CrossRef CAS PubMed.
- K. R. Graham, J. Mei, R. Stalder, J. W. Shim, H. Cheun, F. Steffy, F. So, B. Kippelen and J. R. Reynolds, ACS Appl. Mater. Interfaces, 2011, 3, 1210–1215 CAS.
- C. Melzer, E. J. Koop, V. D. Mihailetchi and P. W. M. Blom, Adv. Funct. Mater., 2004, 14, 865–870 CrossRef CAS.
- V. D. Mihailetchi, J. K. J. van Duren, P. W. M. Blom, J. C. Hummelen, R. A. J. Janssen, J. M. Kroon, M. T. Rispens, W. J. H. Verhees and M. M. Wienk, Adv. Funct. Mater., 2003, 13, 43–46 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: TGA thermograms, cyclic voltammograms, extended solar cell characteristics, GIWAXS line-cuts, FET and SCLC charge carrier mobility. See DOI: 10.1039/c6ra17096j |
| ‡ B. Walker and S. Yum contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |