Impact of reaction variables and PEI/l-cysteine ratio on the optical properties and cytocompatibility of cationic Ag2S quantum dots as NIR bio-imaging probes†
Abstract
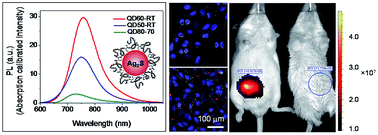
Near-infrared emitting semiconductor quantum dots (NIRQDs) are popular fluorescent probes due to better penetration depth and elimination of tissue autofluorescence. Here, we demonstrate one pot aqueous synthesis of cytocompatible, strongly luminescent, cationic Ag2S NIRQDs utilizing a mixed coating composed of branched polyethyleneimine (PEI)-25 kDa and L-cysteine (Cys) as in vitro luminescent tags and in vivo optical imaging agents. Ultrasmall sizes, a clear first excitonic peak in the absorption spectra, relatively narrow emission peaks with maxima between 730 and 775 nm and a Stokes shift less than 100 nm were obtained. Lifetime measurements indicate excitonic and defect-related emissions. Interestingly, not the emission maxima but the intensity was influenced by the Cys amount more dramatically. PEI/Cys 60/40 mol ratio provided the highest quantum yield reported until now for Ag2S NIRQD (157%) emitting at such a short wavelength. Low molecular weight PEI failed to produce luminescent QDs. Cytotoxicity evaluation of the most strongly luminescing NIRQDs, revealed the PEI/Cys (mol mol−1) 50/50 composition as the non-toxic composition below 2.4 μg Ag per mL concentration. Others had low-toxicity. In vitro microscopy experiments showed endosomal distribution of NIRQDs in Hela cells and strong NIR signal. In vivo imaging study demonstrated that Ag2S NIRQDs could effectively be used as strong optical imaging agents.

 Please wait while we load your content...
Please wait while we load your content...