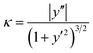Understanding structural characteristics of out-of-register hIAPP amyloid proteins via molecular dynamics†
Inchul Baek‡
,
Myeongsang Lee‡ and
Sungsoo Na*
Department of Mechanical Engineering, Korea University, Seoul 02841, Republic of Korea. E-mail: nass@korea.ac.kr
First published on 9th August 2016
Abstract
Amyloid oligomers are implicated in several neurodegenerative diseases; studies have shown oligomeric amyloids form fibrillary amyloids and have toxic effects on cell function. Several experimental and computational studies have investigated in-register amyloids and their characteristics. However, recently, out-of-register amyloid structures have been observed and their inherent weak structural stability exhibits higher toxicity under physiological conditions compared to that of in-register amyloids. Specifically, by varying the size of oligomeric hIAPP out-of-register structures from 4 layers to 20 layers, we successfully analyzed the structural characteristics of fibrillary out-of-register hIAPP; the critical structure size of out-of-register hIAPP is related to fibrillar growth from protofibrils. Through the structural analysis of out-of-register hIAPP, we shed light on the fibrillar growth mechanism of out-of-register hIAPP oligomer in detail.
Introduction
Amyloid proteins have pathological implications for several degenerative diseases.1–3 These amyloid proteins have toxic characteristics and disrupt normal cell function in the human body.4,5 For example, fibrils of Aβ amyloid, α-synuclein and tau proteins are frequently deposited in the human cerebrum, which disrupts normal functions and causes neuro-degenerative diseases such as Alzheimer's, Parkinson's, and Huntington's diseases as well as transmissible spongiform encephalopathies.6 Human islet polypeptide (hIAPP) and β2-microglobulin also induce degenerative diseases like type II diabetes and dialysis-related diseases.7–10 Specifically, amyloid oligomers have toxic characteristics not only as deposits inside cells, but they can also permeate lipid bilayers of membranes.11,12 After lipid bilayer permeation and removal by amyloid oligomers, ion-channels inside membranes are generated and abnormal physiological phenomena occur. Furthermore, amyloid oligomers also act as seeds that can grow into fibrillary amyloids.13 Thus, understanding amyloid oligomers is important to prevent their toxic behavior and their growth into fibrillary amyloids.The structural characteristics of amyloid oligomers and their stabilities have been investigated through experimental techniques and computational methods. For example, experiments by Collins et al., showed that fibrillary amyloids were grown by repetitive fragmentation and elongation mechanisms.14 Among of them, fragmented amyloid oligomers are important due to their toxic characteristics and their role as seeds for growth into fibrillary amyloids. According to Xue et al., fragmented amyloid fibrils show enhanced toxicity compared to fibrillary amyloids.15 They also examined the growth of fragmented amyloids into additional fibrillary amyloids. So, to understand the structural characteristics of amyloid oligomers, Shea, Horn, Na and Yao groups investigated the effects of different sizes of amyloid oligomers using molecular dynamics (MD) methods, respectively.16–19 They reported the stabilities of amyloid oligomers by analyzing root mean square fluctuations (RMSF), binding energies, and the number of hydrogen bonds. They reported that the different sizes of amyloid oligomers had varying structural stabilities, and determined which sizes of amyloid oligomers are related to toxic behavior or growth to fibrils. One common structural characteristic of amyloid oligomers is that they consist of cross-β structures with steric zipper compositions.20–22 According to Sawaya et al., polymorphic amyloid proteins (i.e. Sup35, tau, insulin and Aβ) exist and they share the cross-β forms with steric zipper.20 Esposito et al. also reported that the amyloid fibrils and oligomers consist of cross-β and steric zippers with dry interfaces.23 Thus, based on these cross-β zipper characteristics of amyloids oligomers which are related to toxic behavior and growth into amyloid fibrils, understanding the cross-β zipper types of amyloid oligomers has been studied.
For the polymorphic hIAPP22–29 oligomer experimental study, Nielsen et al. and Madine et al. found polymorphic hIAPP amyloid fibrils using solid state-nuclear magnetic resonance (ss-NMR).21,24 Analysis of polymorphic hIAPP22–29 oligomers (i.e. protofibrils) at the atomic scale was also performed using computational methods investigated. According to Na's and Harris's group, the cross-β zipper structures with polymorphic characteristics alter the structural conformation, structural characteristics and their mechanical characteristics as determined by MD and steered molecular dynamics (SMD).25–30 From their results, considerable changes in mechanical properties and stabilities of polymorphic hIAPP22–29 were observed due to hydrogen bonds, hydrophobicity and steric zipper effects. Similarly, for the Aβ protein, Hansmann group reported the different stabilities of polymorphic Aβ16–22 oligomers with cross-β zipper by different hydrophobicity effects.31 Lee et al. investigated the effects of tau protein mutation and found that mutating lysine residues of tau oligomer sustaining cross-β zippers causes changes in the structural bending conformation and stabilities via changes in conformational energies.19 Additionally, to study the effect of charge on amyloids, capping on amyloid proteins has been conducted in both computational and experimental studies. For example, Andreasen et al. reported that capping on hIAPP22–29 alter the structural formation compared to uncapped hIAPP22–29 proteins as seen via MD, transmissible electron microscopy (TEM) and atomic force microscopy (AFM).32 Lee et al. also investigated the effect of capping TTR105–115 amyloid proteins and found that capping alter structural formation as well as structural and mechanical characteristics.33,34 They found that both the number of hydrogen bonds and electrostatic energies of the molecules can alter structural stability. From these types of studies of cross-β amyloid studies considering various factors, cross-β types of amyloid had considerable structural stabilities under various conditions.
Recently, the Eisenberg group examined the different forms of amyloids using experimental tools that revealed amyloid forms that are different from previously reported types (i.e. cross-β zippers and in-register).35 They called the recently revealed amyloids as out-of-register structures. These out-of-register structures showed weak hydrogen bond compositions and higher toxicity than the previously reported in-register cross-β zippers. Normally, in-register cross-β zipper structures are composed of face-to-face β-strands without composing degree angle. However, out-of-register structures had face-to-face β-strands composed with an 80 degree crossing angle. Due to the different face-to-face β-strands and weak hydrogen bonds compositions of out-of-register amyloids, they are more easily ruptured than in-register structures. The Eisenberg group reported several kinds of out-of-register amyloids such as, Aβ, IAPP, hIAPP, β2-microglobulin and tau.35 Considering that oligomer amyloids have a toxic effect on normal cell function and can form amyloid fibrils, it is important to understand these out-of-register amyloid oligomers at an atomic scale in detail.
In this study, we investigated the out-of-register hIAPP22–29 amyloid protofibrils using equilibrium MD simulations. Through the number of hydrogen bonds, molecular-mechanics/Poisson–Boltzmann Surface Analysis (MM/PBSA), and RMSF analysis, we found out that out-of-register structures were morphologically stable, which can be compared to previous simulation results of hIAPP amyloids. We also analyzed the effect of various sizes of out-of-register hIAPP22–29 amyloid proteins to understand the critical protofibril size of these amyloids. Moreover, we investigated the instability of out-of-register amyloids over the critical and particular size by analyzing the curvature of out-of-register hIAPP amyloids. Additionally, structural characteristics like solvent-accessible surface area (SASA) and the number of hydrogen bonds also affect the instability of hIAPP protofibrils over the critical length size. By comparing the previous computational in-register hIAPP22–29 amyloids studies, we found that the role of out-of-register structures have lower structural stability, which can explain the toxic behavior of amyloids.
Material and methods
1. Construction of out-of-register hIAPP protofibrils
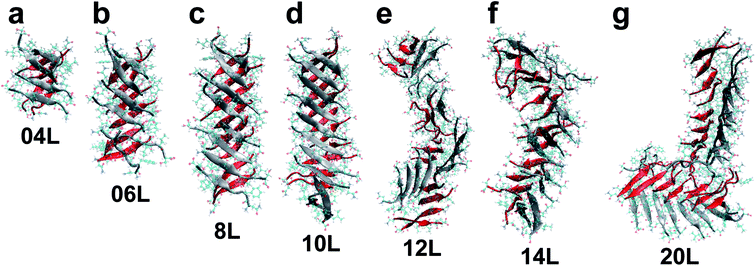
From the out-of-register amyloids reported by the Eisenberg group (i.e. tau, IAPP, β2m and Aβ), there is no hydrophobic rich region in the out-of-register model with a crossing angle. Here, we a used NFGAILS segment from the full-length hIAPP structure based on solid-state nuclear magnetic resonance (ss-NMR) results to compare the previous computational studies of in-register hIAPP structures. Based on NFGAILS segments, we constructed fibrillary out-of-register (OOR) amyloid proteins of the different lengths using protein data bank (PDB) ID of 2KIB. The given structure of 2KIB is composed of eight beta-strands, and we choose two beta-strands to construct OOR hIAPP protofibrils from eight beta-strands. The basic structure of the OOR hIAPP protofibril has a ∼100° crossing angle and no water molecules between each facing β-sheets (Fig. 1a). From previous computational amyloid studies, it is important to find which specific length of fragmented oligomeric amyloids is related to fibrillary amyloid formations. Here, we built OOR hIAPP protofibril model of various lengths that are 4, 6, 8, 10, 12, 14, or 20 layers to evaluate molecular stability and structural characteristics of OOR hIAPP fibrils (Fig. 1b). Here, we constructed the seven types of OOR fibrillary hIAPP amyloids, denoted as 04L, 06L, 08L, 10L, 12L, 14L and 20L with ∼0.48 nm distance between each layers.24,35 During the construction of OOR hIAPP protofibrils, they have a fully constructed hydrogen bonds (H-bonds) region at an intra-basic-structure part (called strong interface) and a partially developed H-bonds region at inter-basic-structure (called the weak interface) (Fig. 1c).2. Equilibration molecular dynamics (MD) simulations
In this study, equilibration molecular dynamics (MD) simulations were performed using GROMACS 4.6.5 with a CHARMM27 molecular force field. All protein structures were packed in dodecahedron simulation box with TIP3P water molecules with 1.5 nm size of thickness.36 The simulation box was electrically neutralized and salt concentration was set to 0.12 M to mimic physiological condition using Na+ and Cl− ions. The internal energy of simulation boxes was minimized using the steepest descent algorithm to remove bad atomic contact in structures. The pressure and temperature of the system were set to 1 bar and 310 K using Parrinello–Rahman pressure coupling and V-rescale temperature coupling method, which can be called the NPT ensemble. To apply the NPT ensemble, a simulation box was sustained with periodic boundary condition. After that, equilibration MD simulations were carried out using a leap-frog algorithm. Three independent 50 ns equilibration simulations were performed for a total 150 ns with 2 fs time step for each model. For the analysis of the structural stability and characteristics of oligomeric OOR hIAPP protofibrils, trajectory and energies were recorded every 2 ps.Result and discussions
1. Conformational results of out-of-register hIAPP protofibrils
After 50 ns equilibration MD simulations, we found that 04L, 06L, 08L and 10L (04–10L) models successfully constructed without any defect in overall structure (Fig. 2a–d and S1–S4a†), whereas the 12L and 14L models considerably changed with conspicuous bending (Fig. 2e and f, S5a and S6a†). Specifically, during equilibration simulation, the 20L model was fractured at the center of the fibrillary structures and one divided part was adhered to the side of another divided part and made an L shape (Fig. 2g). Due to this L conformation of the 20L OOR hIAPP protofibril, the structural conformation and stability analysis of the 20L model was not appropriate to compare with other equilibrated models. Therefore, we excluded the 20L model for analysis of stability characteristics. As shown in Fig. 2, the conformational stability of the equilibrated OOR hIAPP protofibrils suddenly dropped after the 10L model, which has a length of 4.70 nm. At models longer than 10L, we found structural instability in the 12L and 14L oligomer models through their bent conformation, which was accompanied with protofibril breaking and increased length.To support the conformational stability of fibrillary OOR hIAPP amyloids, we calculated the average protofibril curvature to quantifying protofibrils bends (Fig. 3). Curvature of protofibrils was calculated from the last 5 ns of the entire equilibrium simulation with 10 ps intervals. The center point of each basic structure was given as a reference point of protofibril geometry and was used to suggest the geometry function of fibril via Lagrange interpolating polynomial method.37 If the geometry of OOR fibril is given as polynomial function dependent to coordinate x, i.e. y = f(x), then the curvature κ is defined as
The measured curvature indicates the geometry of protofibril deviation from the initial straight conformation of OOR hIAPP oligomer amyloids. For the curvature results, the curvature of 04L and 06L models were omitted because their curvature value were zero, whereas the curvature of the 20L model was also excluded due to the structure fracture after equilibrium MD simulation. The curvature behavior of fibrillary OOR hIAPP amyloids was observed when protofibrils length reached 8 layers (i.e. 3.75 nm). After the 08L model, curvature values of OOR hIAPP protofibril increased by 10 times when two layers of OOR hIAPP were added. This means that as the protofibril curvature increases in length, the OOR hIAPP amyloid protofibrils undergo more bending, which increases flexibility and instability.
These conformational flexibility of instability results can be compared to those in our previous study on different sized-tau proteins where we revealed that in models developed over the specific model (i.e. 10 layers model for WT and Q2K), structural instabilities were observed in the conformations, root mean square deviation (RMSD), and radius of curvature.19 Furthermore, Kahler et al., who reported on the structural characteristics of the Aβ amyloid oligomer and fibrils with respect to different sizes in an MD study, revealed that the specific length size of Aβ oligomer is related to growth of fibrillar amyloids.17 In spite of out-of-register amyloid protofibrils applied in this study is different to previous in-register tau and β-turn-β Aβ amyloid studies, our equilibrated OOR hIAPP protofibrils from MD simulation results have reliable conformational result tendencies along to different length sizes.
2. Conformational feature of out-of-register hIAPP protofibrils
From OOR hIAPP oligomer conformational results and curvature analysis, we investigated the transition of OOR hIAPP protofibrils from straight to bent in the 10L model. We also measured the RMSD and calculated the average RMSF to analyze transition behavior of OOR hIAPP protofibrils as shown in Fig. 4. For the calculation of RMSD and RMSF, we used the g_rms and g_rmsf function in GROMACS every 10 ps. As seen in Fig. 4a, we computed the RMSD values of first simulation for each structure; the measure of the average distance between the atoms of superimposed proteins from the initial structures. Remaining trials of RMSDs are represented at ESI (Fig. S1–S6b†). From the RMSD results, values of 04L, 06L, and 08L (04–08L) models saturated very quickly after 8 ns, and the RMSD value of the 10L model quickly saturated after 10 ns, much later than 04–08L models. On the other hand, 12L and 14L models show late saturation of RMSD time after 25 ns, and even that RMSD value range was considerably larger than the 04–10L models. We also determined that the structures of OOR hIAPP protofibrils are stable up to 10L models. However, when fibril length is longer than over the 10L model, structural stabilities rapidly decrease. These RMSD results could be compared to previous conformation and curvature value results, for which the bend phenomenon was observed after the 10L model.For a more detailed analysis, we measured the average RMSF value of each structure (Fig. 4b) during the last 5 ns of the equilibration simulation. Remaining trials of RMSFs are represented at ESI (Fig. S1–S6c†). From the RMSF results, we decomposed the RMSF values for each residue region in order to analyze the structural stability factor in detail. We found small RMSF values at central section residues (G, A, I) and large values at outer section residue (N, F, L, S) of the fibril axis. Specifically, the RMSF of 04–10L models show similar values, but the RMSF values of 12L and 14L models were higher. Taking this into consideration, 04–10L structures have better structural stability than 12L and 14L structures. These RMSF results could be compared to experimental OOR IAPP studies and computational in-register IAPP studies from dimer to 16-mer. Soriaga et al. recently investigated the crystal structures of OOR IAPP using X-ray diffraction and deposited the PDB data (5E5V).39 Here, they observed the temperature factor of OOR IAPP beta sheets, which exhibits similar data to our average RMSF data of 04–10L models (Fig. S7†). Guo et al. investigated the structural stabilities of various sized in-register IAPP oligomers.16 They observed low RMSF values at the central section residues. They also observed the larger RMSF value in multiple chain composition than monomers. Even though our out-of-register compositions are different from this previous study based on amino acid sequences (N, F, G, A, I, L, and S), our RMSF residue per residue analysis is comparable to previous study in terms of weakness of out-of-register structures. Similarly, RMSF of central section residues and outsides residue could be compared to our previous TTR amyloid protofibril study, where TTR amyloid protofibrils are composed of rich hydrophobic residues.33
Therefore, we found that OOR hIAPP protofibrils are stable up to 10L. After protofibril growth to 10 layers, structural stability rapidly decreased. From RMSD and RMSF graphs, we can determine the critical length of OOR hIAPP protofibrils to be 10 layers, which is about 4.70 nm.
3. The structural characteristics of out-of-register amyloid protofibrils
From the analyses of curvatures conformation, RMSD, and RMSF, we revealed that 10 layers is the critical size for conformational stability. We additionally analyzed the structural stability of OOR hIAPP protofibrils for the number of hydrogen bonds (H-bonds) and solvent-accessible surface area (SASA). From previous computational MD studies of structural stability characteristics could be determined through the H-bonds and SASA values.19,33,40–42 H-bonds plays an important role in determining the three-dimensional structures of proteins and are the main factor for verifying the stability of non-covalently composed of amyloids, acting like chemical glue.43–45 Further, SASA is measures protein exposure to external solution and indicates structure hydrophobic defects. Low hydrophobicity means less effect of solvents.Based on these structural stability characteristics measurements, we checked the H-bonds and SASA of the entire OOR hIAPP structure to verify protofibril stability by different lengths (Fig. 5). To calculate the H-bonds and SASA, we measured the average value of H-bonds and SASA using g_hbond and g_sas tools in GROMAS. Averaged H-bonds and SASA were obtained from the last 5 ns of equilibration simulation with every 10 ps time step. Previous computational studies of amyloids with varying lengths showed that the number of H-bonds was proportional to structural stability size increment, and beta-strand quantity, whereas SASA is inversely proportional to structural stability.41,46,47 The respective proportionality and inverse proportionality of H-bonds and SASA different oligomer length scale were confirmed in our OOR hIAPP oligomer study. As shown in Fig. 5, we found that the H-bonds are increased along with increment of length sizes, whereas SASA areas decreased with decreased lengths. Specifically, the dashed red line is linear proportion line that predicts the proportions of 04–10L model of H-bonds and SASA values. As seen in Fig. 5, the H-bonds and SASA data of 04–10L models are well fitted to the red guided line. For the 12–14L models, we found that number of H-bonds of 12L and 14L models is smaller than predicted by the red line, whereas SASA values were larger than predicted by the red.
Specifically, as shown in Fig. 5a, the quantity of H-bonds constantly increased from 32 in the 04L model to 87 in the 10L model, but H-bonds of 12L and 14L models are not consistently increased with differences of ∼9. In addition, the SASA area also increases constantly from 40.67 nm2 in the 04L model to 86.10 nm2 in the 10L model, whereas 12L and 14L models of OOR hIAPP protofibrils are not with ∼7 nm2 differences as predicted by the red line. The decreased of H-bonds between 12L and 14L models implies that the number of hydrogen bonds is decreasing for each size model, which induces a lack of coherence and instability of OOR hIAPP protofibrils. Furthermore, the larger SASA of 12L and 14L compared to the red line also tells that there are defects in the structure of these protofibrils as well as related to curvedness of structure. These structural features of the H-bonds and SASA could be confirmed through the related computational study of tau and Aβ amyloids. Furthermore, we found a decreased number of H-bonds in fibrillary OOR hIAPP amyloids than for in-register hIAPP protofibrils, which indicates less stability of OOR hIAPP protofibrils compared to in-register structures in terms of having toxic characteristics. In summary, when OOR hIAPP fibrils grew to greater than 10 layers or 4.70 nm, defects in OOR hIAPP protein structure were evident, leading to structural collapse and instability.
4. Binding energy of out-of-register amyloid protofibrils
To support the structural feature (i.e. H-bonds and SASA) of fibrillary OOR hIAPP, we calculated binding free energy between each basic-structure with inter-basic-structure region composed with weak interface. To determine the binding energy coherence of OOR hIAPP protofibrils, we used the g_mmpbsa tool of GROMACS from the last 5 ns of equilibrated simulation with every 10 ps time step.48 Considering the amyloids are mainly composed of non-covalent compositions, less binding free energy has more driving force to each protein segment and stabilized energy between each atom. Here, the binding free energy is sum of the solvation free energy and molecular mechanics potential energy (MM energy). The solvation free energy is the sum of polar solvation energy, the interaction between protein and solvents via electrostatic-interactions, and non-polar solvation energy, the hydrophobic characteristics. We computed binding energy from equilibrated sections and calculated averages of binding free energy.We found that the coherence of binding free energy could be found from 04L to 14L models as shown in Table 1, which agree with the RMSD, RMSF, curvature, SASA, and H-bonds results. From the 04L to 14L models, we can see the smallest polar solvation energy at 04L, non-polar solvation energy at 08L, and the smallest MM energy at 08L. The total binding energy of 08L had the smallest value in among all length models. This stable binding energy of 08L seem to be caused by the end β-strand fluctuation of 10L model as shown in Fig. 2. As shown in Fig. 2d, lower β-strands region are severely detached from 10L model. Furthermore, compared to previous MM/PBSA results of amyloid oligomer or fibrillar studies, our MM/PBSA energies are relatively higher than previous studies results,26,40,41,49 which means higher structural instability of out-of-register and explain the toxic characteristics. Therefore, 08L model has best structure adherence and stability among the models. This phenomenon was compared to findings of previous various size of amyloid studies of in-register, tau, Aβ and hIAPP.16,17,19
| Model | Epolar (kcal mol−1) | Enon-polar (kcal mol−1) | EMM (kcal mol−1) | Etotal (kcal mol−1) |
|---|---|---|---|---|
| 04L | 194.0 | −25.7 | −411.2 | −243.0 |
| 06L | 219.5 | −25.8 | −433.9 | −240.3 |
| 08L | 217.4 | −27.5 | −440.8 | −250.9 |
| 10L | 195.6 | −26.4 | −396.2 | −226.9 |
| 12L | 199.2 | −24.7 | −403.4 | −228.9 |
| 14L | 222.3 | −25.2 | −429.1 | −232.0 |
We found that the OOR hIAPP oligomer has critical conformational stability length at 10 layers (i.e. 4.70 nm length) among the models examined. Specifically through the MM/PBSA analysis, if oligomeric hIAPP protofibrils were grown more than 3.75 nm, the binding energy of structures suddenly collapsed and structural stability decreased. In this state, over the specific length model of protofibrils, amyloids break, which could be connected to fibrillar oligomer mechanism through fragmentation and elongation or ‘stop and go’.50 Overall, through the calculation of binding free energy, we could support the H-bonds and SASA data and understand the structural characteristics of fibrillary OOR hIAPP amyloids.
Conclusions
In this study, we investigated the fibrillary OOR hIAPP amyloids with respect to various sizes in detail using MD simulations. From these results, we found that structural conformations of oligomeric OOR hIAPP models larger than 08L (3.75 nm) adopt a bent shape and exhibit structural instability. Through analysis of the curvature, RMSD and RMSF, we supported the conformational results. Moreover, by analyzing the H-bonds, SASA and binding free energies, our results supported the critical length size of oligomeric OOR hIAPP amyloids. By analyzing the OOR oligomeric hIAPP amyloids in detail, we not only shed light on structural characteristics of out-of-register amyloids in detail, but also provided the useful information about structural instability of out-of-register amyloids compared to reported in-register amyloids in detail.Acknowledgements
S. N. gratefully acknowledges the Basic Science Research Program, through the National Research Foundation of Korea (NRF) and is funded by the Ministry of Science, ICT & Future Planning (MSIP) (Grants No. 2014R1A2A1A11052389).References
- M. B. Pepys, Annu. Rev. Med., 2006, 57, 223–241 CrossRef CAS PubMed.
- G. Merlini and V. Bellotti, N. Engl. J. Med., 2003, 349, 583–596 CrossRef CAS PubMed.
- D. Eisenberg and M. Jucker, Cell, 2012, 148, 1188–1203 CrossRef CAS PubMed.
- C. Haass and D. J. Selkoe, Nat. Rev. Mol. Cell Biol., 2007, 8, 101–112 CrossRef CAS PubMed.
- F. Chiti and C. M. Dobson, Annu. Rev. Biochem., 2006, 75, 333–366 CrossRef CAS PubMed.
- C. Soto, Nat. Rev. Neurosci., 2003, 4, 49–60 CrossRef CAS PubMed.
- N. M. Kad, S. L. Myers, D. P. Smith, D. Alastair Smith, S. E. Radford and N. H. Thomson, J. Mol. Biol., 2003, 330, 785–797 CrossRef CAS PubMed.
- N. M. Kad, N. H. Thomson, D. P. Smith, D. A. Smith and S. E. Radford, J. Mol. Biol., 2001, 313, 559–571 CrossRef CAS PubMed.
- A. S. DeToma, S. Salamekh, A. Ramamoorthy and M. H. Lim, Chem. Soc. Rev., 2012, 41, 608–621 RSC.
- R. P. R. Nanga, J. R. Brender, J. Xu, G. Veglia and A. Ramamoorthy, Biochemistry, 2008, 47, 12689–12697 CrossRef CAS PubMed.
- J. R. Brender, D. L. Heyl, S. Samisetti, S. A. Kotler, J. M. Osborne, R. R. Pesaru and A. Ramamoorthy, Phys. Chem. Chem. Phys., 2013, 15, 8908–8915 RSC.
- S. A. Kotler, P. Walsh, J. R. Brender and A. Ramamoorthy, Chem. Soc. Rev., 2014, 43, 6692–6700 RSC.
- J. D. Harper and P. T. Lansbury, Annu. Rev. Biochem., 1997, 66, 385–407 CrossRef CAS PubMed.
- S. R. Collins, A. Douglass, R. D. Vale and J. S. Weissman, PLoS Biol., 2004, 2, e321 Search PubMed.
- W.-F. Xue, A. L. Hellewell, W. S. Gosal, S. W. Homans, E. W. Hewitt and S. E. Radford, J. Biol. Chem., 2009, 284, 34272–34282 CrossRef CAS PubMed.
- J. Guo, Y. Zhang, L. Ning, P. Jiao, H. Liu and X. Yao, Biochim. Biophys. Acta, Gen. Subj., 2014, 1840, 357–366 CrossRef CAS PubMed.
- A. Kahler, H. Sticht and A. H. C. Horn, PLoS One, 2013, 8, e70521 CAS.
- C. Wu and J.-E. Shea, PLoS Comput. Biol., 2013, 9, e1003211 CAS.
- M. Lee, I. Baek, H. Choi, J. I. Kim and S. Na, Biochem. Biophys. Res. Commun., 2015, 466, 486–492 CrossRef CAS PubMed.
- M. R. Sawaya, S. Sambashivan, R. Nelson, M. I. Ivanova, S. A. Sievers, M. I. Apostol, M. J. Thompson, M. Balbirnie, J. J. W. Wiltzius, H. T. McFarlane, A. O. Madsen, C. Riekel and D. Eisenberg, Nature, 2007, 447, 453–457 CrossRef CAS PubMed.
- J. T. Nielsen, M. Bjerring, M. D. Jeppesen, R. O. Pedersen, J. M. Pedersen, K. L. Hein, T. Vosegaard, T. Skrydstrup, D. E. Otzen and N. C. Nielsen, Angew. Chem., 2009, 121, 2152–2155 CrossRef.
- L. R. Volpatti, M. Vendruscolo, C. M. Dobson and T. P. J. Knowles, ACS Nano, 2013, 7, 10443–10448 CrossRef CAS PubMed.
- L. Esposito, C. Pedone and L. Vitagliano, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11533–11538 CrossRef CAS PubMed.
- J. Madine, E. Jack, P. G. Stockley, S. E. Radford, L. C. Serpell and D. A. Middleton, J. Am. Chem. Soc., 2008, 130, 14990–15001 CrossRef PubMed.
- M. Lee, H. J. Chang, D. Kim, Y. Lee, H. Suh, N. Ahn, G. Yoon and S. Na, Biophys. Chem., 2015, 199, 1–8 CrossRef CAS PubMed.
- G. Yoon, M. Lee, J. I. Kim, S. Na and K. Eom, PLoS One, 2014, 9, e88502 Search PubMed.
- M. Lee, I. Baek, H. J. Chang, G. Yoon and S. Na, Chem. Phys. Lett., 2014, 600, 68–72 CrossRef CAS.
- J. I. Kim, M. Lee, I. Baek, G. Yoon and S. Na, Phys. Chem. Chem. Phys., 2014, 16, 18493–18500 RSC.
- H. Ndlovu, A. E. Ashcroft, S. E. Radford and S. A. Harris, Beilstein J. Nanotechnol., 2013, 4, 429–440 CrossRef PubMed.
- H. Ndlovu, A. E. Ashcroft, S. E. Radford and S. A. Harris, Biophys. J., 2012, 102, 587–596 CrossRef CAS PubMed.
- W. M. Berhanu and U. H. E. Hansmann, Protein Sci., 2012, 21, 1837–1848 CrossRef CAS PubMed.
- M. Andreasen, K. K. Skeby, S. Zhang, E. H. Nielsen, L. H. Klausen, H. Frahm, G. Christiansen, T. Skrydstrup, M. Dong, B. Schiøtt and D. Otzen, Biochemistry, 2014, 53, 6968–6980 CrossRef CAS PubMed.
- M. Lee and S. Na, ChemPhysChem, 2016, 17, 425–432 CrossRef CAS PubMed.
- M. Lee, H. Choi and S. Na, J. Nanomater., 2016, 2016, 10 Search PubMed.
- C. Liu, M. Zhao, L. Jiang, P.-N. Cheng, J. Park, M. R. Sawaya, A. Pensalfini, D. Gou, A. J. Berk, C. G. Glabe, J. Nowick and D. Eisenberg, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 20913–20918 CrossRef CAS PubMed.
- H. J. C. Berendsen, D. van der Spoel and R. van Drunen, Comput. Phys. Commun., 1995, 91, 43–56 CrossRef CAS.
- S. C. Chapra and R. P. Canale, Numerical methods for engineers, McGraw-Hill, New York, 2012 Search PubMed.
- P. V. O'neil, Advanced engineering mathematics, Cengage learning, 2011 Search PubMed.
- A. B. Soriaga, S. Sangwan, R. Macdonald, M. R. Sawaya and D. Eisenberg, J. Phys. Chem. B, 2016, 120, 5810–5816 CrossRef CAS PubMed.
- H. J. Chang, I. Baek, M. Lee and S. Na, ChemPhysChem, 2015, 16, 2403–2414 CrossRef CAS PubMed.
- Y. Gwonchan, L. Myeongsang, K. Kyungwoo, K. Jae In, C. Hyun Joon, B. Inchul, E. Kilho and N. Sungsoo, Phys. Biol., 2015, 12, 066021 CrossRef PubMed.
- H. Choi, M. Lee, H. S. Park and S. Na, RSC Adv., 2016, 6, 52236–52247 RSC.
- T. P. Knowles, A. W. Fitzpatrick, S. Meehan, H. R. Mott, M. Vendruscolo, C. M. Dobson and M. E. Welland, Science, 2007, 318, 1900–1903 CrossRef CAS PubMed.
- T. P. J. Knowles and M. J. Buehler, Nat. Nanotechnol., 2011, 6, 469–479 CrossRef CAS PubMed.
- M. J. Buehler and Y. C. Yung, Nat. Mater., 2009, 8, 175–188 CrossRef CAS PubMed.
- H. Choi, H. J. Chang, Y. Shin, J. I. Kim, H. S. Park, G. Yoon and S. Na, RSC Adv., 2015, 5, 49263–49269 RSC.
- R. Paparcone and M. J. Buehler, Biomaterials, 2011, 32, 3367–3374 CrossRef CAS PubMed.
- R. Kumari, R. Kumar and A. Lynn, J. Chem. Inf. Model., 2014, 54, 1951–1962 CrossRef CAS PubMed.
- W. Berhanu and A. Masunov, J. Mol. Model., 2012, 18, 1129–1142 CrossRef CAS PubMed.
- D. Pinotsi, A. K. Buell, C. Galvagnion, C. M. Dobson, G. S. K. Schierle and C. F. Kaminski, Nano Lett., 2014, 14, 339–345 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra19100b |
| ‡ I. B. and M. L. made equally contribution to this work. |
| This journal is © The Royal Society of Chemistry 2016 |