Block copolymer and organic salts in forming aqueous biphases: a platform to identify molecular interactions in aqueous medium†
Abstract
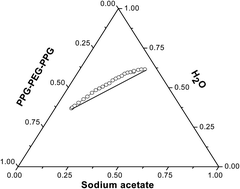
An aqueous solution of a tri-block copolymer PPG–PEG–PPG has been used with two organic salts, sodium acetate and sodium citrate, to create new aqueous biphasic systems (ABS). Well defined phase diagrams with suitable tie-lines were obtained by a turbidometric titration method at 303 K. The tie-line lengths and slopes of the tie lines were calculated and the results were validated using Othmer–Tobias and Bancroft equations. The binodals indicate that sodium citrate offers a larger biphasic region with the polymer than the acetate salt. The aggregation number of the pure polymer solution and that separated from ABS, determined by a fluorescence quenching method, clearly indicate a hike due to reorganization of the polymer chains upon phase separation. The newly designed systems have been used to extract three different vitamins, thiamine (B1), riboflavin (B2) and cholecalciferol (D3), together with the biomolecule caffeine. These studies indicate that caffeine interferes with the extraction of cholecalciferol. Possible interactions between the two biomolecules, caffeine and cholecalciferol, thus identified, were further verified by gathering different spectral evidence like absorption, emission, 1H NMR, and mass spectra and from confocal microscopic studies. Therefore results of binary extractions of two components in an ABS can act as an indicator of molecular interactions much a priori to confirmation by sophisticated analytical techniques.

 Please wait while we load your content...
Please wait while we load your content...