Towards cost-effective silicon anodes using conductive polyaniline-encapsulated silicon micropowders†
Abstract
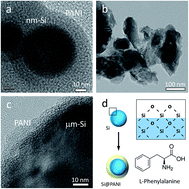
To overcome the remaining issues of nanostructured Si anodes such as compromised packing density and prohibitive cost associated with sophisticated structural designs and synthetic approaches, we investigated the feasibility towards practically viable Si anodes by combining low-cost material precursors with facile and scalable mechanical grinding and solution processes. By encapsulating Si particles in a conductive polyaniline matrix that serves as an effective buffer layer, we demonstrate stable capacities along with enhanced efficiency and volumetric energy output from solar-grade Si granule-derived Si micropowders. A systematic comparative study with nano-sized Si counterparts presents competitive cost weighted performance for the Si micropowder-enabled anode systems, which offers the potential for realizing high-performance, cost-effective lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...