Characterization of depolymerized lignin and renewable phenolic compounds from liquefied waste biomass†
Junfeng Fenga,
Jianchun Jiang*ab,
Zhongzhi Yanga,
Qiuli Sua,
Kui Wangac and
Junming Xu*c
aInstitute of Chemical Industry of Forest Products, Chinese Academy of Forestry, National Engineering Laboratory for Biomass Chemical Utilization, Key Laboratory on Forest Chemical Engineering, Jiangsu Province, Nanjing 210042, China. E-mail: fengjunfeng0104@163.com; Fax: +86 25 85482478; Tel: +86 13770663924
bJiangsu Qianglin Biomass Energy Co., Ltd., Liyang 213364, China. E-mail: bio-energy@163.com; Fax: +86 25 85482488; Tel: +86 13951039428
cResearch Institute of Forestry New Technology, Chinese Academy of Forestry, No. 1, Xiangshan Village, Haidian District, Beijing 100091, China. E-mail: lang811023@163.com
First published on 3rd October 2016
Abstract
This investigation aimed to analyze the renewable phenolic compounds that separate from liquefied mason pine. One-step thermal conversion of biomass to phenolic products from waste mason pine using an acidic catalyst and methanol was accomplished under mild conditions. Three fractions (fractions 1#, 2#, and 3#) of phenolic compounds with high added-value were extracted with water–organic solvent from liquefied oil via a stepwise fractionation process. The structural features of three phenolic compounds and depolymerized lignin were analyzed and identified by a combination of heteronuclear single quantum correlation-nuclear magnetic resonance, gel permeation chromatography, Fourier transform infrared spectroscopy, and thermogravimetric analysis, showing interesting functionalities for biochemical and biofuel applications. In this investigation, guaiacyl (G) and p-hydroxybenzoate (PB) aromatics were the basic units of three phenolic compound fractions. Etherified syringyl aromatics were evident in fractions 1# and 3#. There were only single-aromatic-ring units (such as G and PB units) in phenolic compound fraction 2#. In the aromatic region, the absence of β-O-4′ ether linkage, resinol, and phenylcoumaran units in three phenolic compound fractions indicated that depolymerization of lignin occurred during the liquefied biomass process. The molecular weights of three phenolic compound fractions were significantly different (797, 249, and 497, respectively) along with fractions 1#, 2#, and 3#. The phenolic compounds could be separated into a high, lower, and lowest molecular weight fraction in this study. As evidenced by GC-MS spectra, the three phenolic compound fraction products and depolymerized lignin were mainly comprised of phenolic derivatives, such as 3-methyl-4-ethylphenol, 4-ethyl-2-methoxyphenol, and 3-methylcatechol. Percentages of the total phenols and derivatives in the three phenolic compound fractions and depolymerized lignin were 77.59%, 81.76%, 80.19%, and 78.86%, respectively. Therefore, the three phenolic compound fractions were clearly quantified and valuable, and can be used as chemical products.
1. Introduction
With increasing fuel demands and growing concern for the effects of greenhouse gas emissions from fossil fuels, developing renewable and sustainable chemical and fuel production methods is imperative. Lignocellulosic biomass is one of the most promising renewable energy sources for the sustainable production of chemicals and fuels.1,2 Unlike starch and corn, biomass is inedible, and its use does not have a direct negative impact on food supply. Waste lignocellulosic biomass such as agricultural residues, paper-making waste, and furniture manufacturing waste are abundant biomasses currently being underutilized.3,4 Indeed, the aforementioned problems have led to intensified efforts in developing lignocellulose-based biochemical and biofuels. These approaches maximize all of the components of lignocellulosic biomass, namely hemicellulose, cellulose and lignin. The valorized natural lignin component, however, has received little attention,5 especially relative to hemicellulose and cellulose, partly because of its structural complexity, relative thermal stability, and biological recalcitrance.Lignin, a natural amorphous three-dimensional aromatic polymer consisting of methoxylated basic phenylpropane structures, confers rigidity and strength to biomass and is the main constituent that protects hemicellulose and cellulose from microbial attack.6–8 Although the composition of lignin varies considerably with the lignocellulosic biomass, particularly in terms of the quantity and type of polymer linkages and the number of methoxy units in the aromatic rings, the lignin used for energy production typically contains about 35–45% lignocellulosic biomass. Therefore, it has enormous potential in supporting biochemical and biofuel requirements.8–11 As little as 2% of lignin is used in low-value products such as dispersants or binding agents, with the remainder burned as low-value fuel.12 Because of lignin's unique chemical structure, a wide variety of feedstock and fine chemicals, particularly aromatics and fuels, can be obtained from it. The appropriate catalytic technology for utilizing lignin should be developed.
Thermochemical methods, which include solvent liquefaction and fast pyrolysis, can convert solid biomass into liquid bio-oil.13 Orientation-pressurized liquefaction, for example, can be an efficient means of converting biomass with high lignin content into liquefied oil. The liquefied oil or bio-oil is a complex liquid that contains more than 200 different compounds. Previous studies14,15 have separated the liquefied oil with water into water-soluble and water-insoluble fractions. The water-soluble fraction mainly contains sugars, anhydro-sugars, furfural, hydroxyacetaldehyde, hydroxyacetone, alcohols, and esters, which can be hydrogenated and converted into high added-value chemicals. Less is known about the water-insoluble fraction and depolymerized lignin. This fraction mainly consists of phenolic compounds derived from thermal pyrolysis of lignin in lignocellulosic biomass. Depolymerized lignin is difficult to hydrogenate because of the rapid polymerization of its high molecular-weight aromatic compounds. Depolymerized lignin from liquefied oil is mostly not volatile, which makes gas chromatography-mass spectrometry (GC-MS) or pyrolysis gas chromatography-mass spectrometry (Py-GC-MS) impossible without functionalizing it.16 High-performance liquid chromatography-mass spectrometry (HPLC) is possible in principle, but it remains less effective for the complicated mixtures of compounds with many different molecular weights and chemical structures. Spectroscopic techniques such as Laser Raman Spectroscopy (LRS) and Fourier transform-infrared spectroscopy (FT-IR) spectroscopy can give qualitative insight into the functional groups in depolymerized lignin or phenolic compounds.17 Gel permeation chromatography (GPC) can be used to give the approximate molecular weights of depolymerized lignin or phenolic compounds.18 In contrast to the techniques mentioned above, nuclear magnetic resonance (NMR) spectroscopy has the potential of providing comparative and qualitative insight into depolymerized lignin or phenolic compounds. 1H and 13C NMR spectroscopy have been used to identify the primary functionality of depolymerized lignin or phenolic compounds.14,19,20 Two-dimensional heteronuclear single quantum correlation-nuclear magnetic resonance (2D HSQC NMR) spectroscopy has been used to provide highly detailed information about the substructures of lignin in biomass; however, this technique has not been used in the analysis of depolymerized lignin or phenolic compounds. In this investigation, HSQC NMR was used to show the absence of β-aryl ether, phenylcoumaran, and resinol structures in three different fractions containing phenolic compounds.
The objective of this study was to develop a fundamental understanding of the chemistry of phenolic compounds by using HSQC NMR, FT-IR, and GPC. These methods allow the design of highly efficient processes for the production of fuels and chemicals from bio-oils.
In this study, depolymerized lignin from liquefied oil could be easily separated into the three different fractions (fractions 1#, 2#, and 3#) containing renewable phenolic compounds by using a stepwise fractionation process. GPC results indicate that the molecular weights (Mw) of compounds in fractions 1#, 2# and 3# are markedly different (622, 105, and 428, respectively). The fractions were characterized by 2D HSQC NMR and FT-IR spectroscopy. p-Hydroxybenzoate (PB), guaiacyl (G), and syringyl (S), as well as p-hydroxyphenyl (H) aromatics in minor amounts, were the basic units of the phenolic compounds. The natural lignin β-O-4′ ether linkage (A), resinol units (B), and phenylcoumaran (C) units are not present in the phenolic compounds, thus indicating that depolymerization of lignin occurs during the liquefied biomass process. Only single-aromatic-ring units such as G and PB units are present, as evidenced by the HSQC cross-signals of phenolic compounds in fraction 2#. GC-MS results indicate that the three fraction products and depolymerized lignin mainly consist of phenolic derivatives such as 3-methyl-4-ethylphenol, 4-ethyl-2-methoxyphenol, and 3-methylcatechol.
2. Experimental
2.1. Chemicals
Industrial waste biomass from mason pine was collected from a farm in Sichuan (China). It was pulverized to pass through a 60-mesh sieve and then oven-dried at 105 °C overnight. The dried material was stored in a sealed bag until use. Element analysis using an elemental analyzer (Elementar, Vario Micro) showed that the material contained C (49.21%), H (4.35%), O (46.21%), N (0.04%), and S (0.19%). Composition analysis of the mason pine sample, which comprises ash analysis, extraction using a benzene–ethanol mixture, extraction using 1% NaOH, as well as acid-insoluble lignin, cellulose, and hemicellulose analyses were completed according to the standard test methods of ASTM 2007. The percentage of ash in mason pine (based on ASTM D 1102-1984) was 0.33 wt%. The percentage of benzene–ethanol-soluble matter was 3.16 wt%, as determined through a standard test method for benzene–ethanol solubility of wood (ASTM D 1107-1996). The percentage of extract obtained using 1% NaOH solution was 22.87 wt% (ASTM D 1109-1984). Using a standard test method for acid-insoluble lignin in wood (ASTM D 1106-1996), we found that the lignin content was 28.67 wt%. The proportions of cellulose and hemicellulose were 48.86 wt% and 11.53 wt%, respectively, as determined according to the standard method for holocellulose (ASTM D 1103-60) and pentosan in wood (ASTM D 1104-56). All chemicals used were of analytical grade and were commercially available. They were used without further purification.2.2. Analytical methods
Functional groups of phenolic compounds and depolymerized lignin in the three fractions were determined by FT-IR spectroscopy using a Nicolet iS10 (USA). Analysis was completed from 4500 to 500 cm−1 at a resolution of >0.4 cm−1, a wavenumber accuracy of >0.01 cm−1, and an ASTM standard linearity better than 0.1% T.The molecular weights of the phenolic compounds and depolymerized lignin in the fractions were measured by GPC using a Waters 1515 system (USA) equipped with a manually packed column. GPC was performed using 20 μL injection volumes. Analysis was completed in 25 min. Tetrahydrofuran and polystyrene were used as solvent and an internal standard, respectively.
The structures of phenolic compounds and depolymerized lignin in the fractions were determined by NMR spectroscopy. 1H–13C correlation 2D HSQC NMR spectra were recorded on a Bruker DRX 500 NMR spectrometer operated at 500 MHz. The spectral widths for the 13C and 1H dimensions were 120.0 ppm and 8.5 ppm, respectively. The measurements were conducted in dimethyl sulfoxide (DMSO) solvent at 30 °C, and tetramethylsilane was used as an internal reference.
Thermal variations of the phenolic compounds and depolymerized lignin in the fractions were observed under a N2 atmosphere in a thermogravimetric (TG) analyzer (PerkinElmer). The N2 flow rate was kept at about 200 mL min−1 under standard conditions. A 5 mg sample was used. The samples were placed in crucibles, which were then heated at 120 °C and kept for 30 min under a N2 atmosphere to remove all of the water from the phenolic compounds and depolymerized lignin. Afterward, the samples were heated from 30 °C to 300 °C at a heating rate of 10 °C min−1.
2.3. Preparation of depolymerized lignin and the three fractions containing phenolic compounds
The three phenolic compound fractions were obtained from liquefied waste mason pine (process is shown in Fig. S1†). Preparation of the fractions from waste biomass consists of liquefaction and separation. Liquefaction of the biomass involves reaction of 60 g of mason pine powders with 420 g of methanol and 1.5 of g sulfuric acid with 30 min heating at 200 °C in an autoclave. The liquefied product is a yellowish powder consisting of many compounds. After liquefaction, the fractions could be separated from the liquefied products by addition of water and organic solvent and then extraction, centrifugation, and distillation. The depolymerized lignin was also prepared through the same liquefaction reaction. The depolymerized lignin and fractions were kept under refrigeration and analyzed within two weeks of preparation.3. Results and discussion
3.1. HSQC NMR spectroscopy of the phenolic compounds
2D 1H/13C NMR spectroscopy can provide important structural information of complex materials, as it allows the resolution of otherwise overlapping resonances observed in either of 1D 1H or 13C NMR spectroscopy.20,21 To determine the structures of biomass-derived phenolic compounds, as well as the linkages between phenols and the associated side chain groups, the three fractions were analyzed by 2D HSQC NMR spectroscopy in the study.The three fractions contain phenolic monomers and dimers with various functionalities, especially methoxy, aldehyde, ether, ester, methyl, and complex alkyls. The possible substructures and units (A, B, C, I, S, S′, G, PB, and H) of the three fractions are presented in Fig. 1. In detail, the A, B, C, I, S, S′, G, PB, and H were on behalf of β-O-4′ ether linkage, resinol units, phenylcoumaran units, p-hydroxycinnamyl alcohol groups, etherified syringyl units, oxidized syringyl units, guaiacyl units, p-hydroxybenzoate units, and p-hydroxyphenyl units, respectively.
All of the fractions for 2D HSQC NMR spectroscopy were dissolved in DMSO-d6. The main cross peaks of the fractions in HSQC NMR spectra are presented in Table 1. The spectra (Fig. 2) can be divided into three regions, namely, (1) aromatic regions (δC/δH 90–145/4–8.25 ppm), (2) C–O aliphatic side chain regions (δC/δH 50–95/2.75–6.0 ppm), and (3) C–C aliphatic side chain regions (δC/δH 5–50/0.5–3.0 ppm). These three regions are shown in greater detail in Fig. 3a–c.
| Label | δC/δH (ppm) | Assignments |
|---|---|---|
| a A: β-O-4′ ether linkage; B: resinol units; C: phenylcoumaran units; I: p-hydroxycinnamyl alcohol and groups; S: etherified syringyl units; S′: oxidized syringyl units, G: guaiacyl units; PB: p-hydroxybenzoate units; H: p-hydroxyphenyl units. | ||
| –COCH3 | 25.3/1.82–1.68 | Acetyl CH3 |
| Cβ | 53.3/3.46 | Cβ–Hβ in phenyl coumarane units (C) |
| Bβ | 53.5/3.06 | Cβ–Hβ in resinol units (B) |
| –OCH3 | 56.0/3.36, 3.62–3.88 | C/H in methoxyl group |
| Aγ | 59.5–59.7/3.40–3.63 | Cγ–Hγ in β-O-4′ linkage (A) |
| Iγ | 61.4/4.10 | Cγ–Hγ in p-hydroxycinnamyl alcohol and groups (I) |
| Cγ | 62.5/3.73 | Cγ in phenyl coumarane S unit (C) |
| Bγ | 71.0/3.82, 4.18 | Cγ–Hγ in resinol units (B) |
| Aα | 70.5/3.00–3.10 | Cα–Hα in β-O-4′ linkage (A) |
| Aβ(G/H) | 83.9/4.29 | Cβ–Hβ in β-O-4′ linked to G units (A) |
| Bα | 84.8/4.65 | Cα–Hα in resinol units (B) |
| Aβ(S) | 85.9/4.12 | Cβ–Hβ in β-O-4′ linked to S units (A) |
| Cα | 86.8/5.46 | Cα–Hα in phenylcoumaran units (C) |
| S2,6 | 100.0/4.50 | C2,6–H2,6 in etherified syringyl units (S) |
| S′2,6 | 105.1/4.00 | C2,6–H2,6 in oxidized syringyl units (S′) |
| G2 | 110.9/6.98 | C2–H2 in guaiacyl units (G) |
| G5 | 116.0/6.79 | C5–H5 in guaiacyl units (G) |
| G6 | 119.0/6.80 | C6–H6 in guaiacyl units (G) |
| Iβ | 128.2/6.25 | Cβ–Hβ in p-hydroxycinnamyl alcohol and groups (I) |
| Iα | 128.4/6.44 | Cα–Hα in p-hydroxycinnamyl alcohol and groups (I) |
| PB2,6 | 130.0/7.51 | C2,6–H2,6 in p-hydroxybenzoate units (PB) |
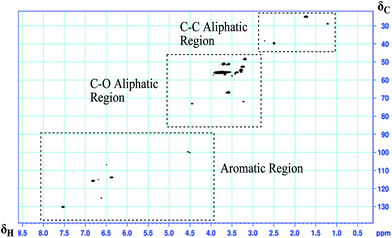 | ||
| Fig. 2 HSQC spectra of fraction 1# with the aromatic, aliphatic C–O, and aliphatic C–C regions highlighted. | ||
Slight changes in the chemical shifts of the aromatic region for the three fractions are similar to reports of cross-signals for lignin.21,23,26 The changes in correlation signals may be due to the interaction of other functional units and groups that form in the biomass liquefaction process i.e., on the altered side chain of the S, S′, G, and PB aromatic rings. The aromatic regions of the three fractions are obviously different. Correlation signals for S′ units are present in fraction 1# only. There are no correlation signals of S′ and S units in fraction 2#. Fraction 3# spectra have a ratio of S units higher than that for fraction 1#. Fraction 3# spectra also have a higher ratio of G units than fractions 1# and 2#. Signals for fraction 3# are much broader than those in fractions 1# and 2#, consistent with its higher molecular weight and slower molecular motion.21 Only PB and G groups were observed in the aromatic region of all three fractions.
Based on these results, a preliminary conclusion can be made that the aromatic units in the three phenolic compounds fractions were primarily S, PB and G with minor amounts of H. No evidence can be found for the formation of poly-nuclear aromatics, such as naphthalene and other polycyclic aromatic hydrocarbons, indicating that such condensations are not prominent during the biomass lignin liquefaction process. Meier proposed several different dimeric substructures and units including diphenyl ether, diphenyl ether, phenylcoumaran (C) and resinol (B).36 In this investigation, characteristic 2D HSQC cross-signals in the aromatic region corresponding to G and PB units were evident in all three phenolic compounds, and S units were evident in fractions 1# and 3#. In particular, there were only single-aromatic-ring units (such as G units and PB unit) from the HSQC cross-signals of phenolic compounds fraction 2#. In the aromatic region, the absence of A, B, and C units in the three phenolic compound fractions indicate that depolymerization of lignin occurs during the liquefied biomass process. Meier proposed the existence of biphenyl units in which the two aromatic rings were linked by a 5-5′-bond.36 This structure has been identified in lignin and has very high bonding dissociation energy among the various interunit linkages. This implied the structure may be retained during the liquefaction process, although it could not be confirmed from 2D HSQC NMR or 1D 13C NMR spectroscopy until now. Biphenyls are not easily detected in 2D HSQC NMR spectra due to the tertiary nature of the diagnostic 5-carbons (without bonded hydrogen). In the natural lignin, biphenyls were readily identified as they existed as dibenzodioxocins that had diagnostic α bonds and converted into volatile monomers and repolymerization after the primary thermochemical conversion.
3.2. FT-IR spectroscopy of depolymerized lignin and phenolic compounds
FT-IR spectroscopy was used to evaluate the structures of depolymerized lignin and three phenolic compound fractions from liquefied waste mason pine (Fig. 4). The assignment of major peaks are summarized in Table 2. Both the depolymerized lignin and three phenolic compound fractions contained carbon–carbon double bonds, hydroxyl, carbonyl groups, ether bonds, and aromatic rings. The liquefaction of waste mason pine was not only holocellulose degrading but also resulted in the decomposition of high molecular polymerization lignin, reactive intermediates with hydroxyl and/or carbonyl group, and carbon–carbon double bonds. The major linkages between the structural units of lignin in waste mason pine are β-O-4′ (β-aryl ether), β–β (B), and β-5 (C). On the other hand, the liquefaction of alkali lignin was mainly polymerization lignin with β-O-4′.| Functional groups | Wave number range (cm−1) | |||
|---|---|---|---|---|
| Fraction 1# | Fraction 2# | Fraction 3# | Depolymerized lignin | |
| a Phenolic compounds including three phenolic compound fractions.b Depolymerized lignin was the product of alkali lignin liquefaction. | ||||
| –OH vibrations | 3420 | 3400 | 3430 | 3450 |
| C–C–H vibrations | 2930, 2850 | 2890, 2750 | 2930, 2830 | 2900, 2800 |
| Carbonyl stretching | 1735 | 1750 | 1700 | 1800 |
| Benzene skeleton vibration | 1600, 1510 | 1600, 1520 | 1650, 1500 | 1650, 1530 |
| C–O–C asymmetric vibration | 1260 | 1200 | 1250 | 1180 |
| C–O stretching | 1050 | 1040 | 1110 | 1050 |
The peaks of the three phenolic compound fractions were consistent (Fig. 4). The obvious peak at around 3400 cm−1 represents the –OH groups from phenols in both depolymerized lignin and phenolic compounds. By analyzing the FT-IR spectra of depolymerized lignin and the three phenolic compound fractions, the peak at approximately 2900 cm−1 and 2800 cm−1 represent C–H in a symmetric stretching of aliphatic methyl and methylene (CH3 and CH2) groups. A band appearing at around 1600 cm−1 was characteristic of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C skeleton vibration in aromatic skeletal of lignin units.37 This peak was an obvious absorption band observed in the three phenolic compound fractions, indicating the depolymerized natural lignin had been concentrated into three phenolic compound fractions successfully after the separation process. Besides, the strong peak at around 1050 cm−1 represents the C–O groups stretching from phenolic compounds, while the same situation is weak in depolymerized lignin.
C skeleton vibration in aromatic skeletal of lignin units.37 This peak was an obvious absorption band observed in the three phenolic compound fractions, indicating the depolymerized natural lignin had been concentrated into three phenolic compound fractions successfully after the separation process. Besides, the strong peak at around 1050 cm−1 represents the C–O groups stretching from phenolic compounds, while the same situation is weak in depolymerized lignin.
3.3. GPC analysis of depolymerized lignin and phenolic compounds
The GPC analysis results of depolymerized lignin and three phenolic compounds fractions from liquefied waste mason pine were shown in Fig. 5. And Table 3 presented the detail data including Mn, Mw, Mp, and Mz results of depolymerized lignin and three phenolic compounds fractions. With the increase of retention time, molecular weights became lower, but the content was getting higher, which showed the form of a continuous distribution in Fig. 5. This result indicated lignin component in the waste mason pine had effective degraded in small molecules phenols and phenol derivatives during the liquefaction process.| Compounds | Retention time (min) | Mn | Mw | Mp | Mz |
|---|---|---|---|---|---|
| Fraction 1# | 13.814 | 278 | 797 | 428 | 5735 |
| Fraction 2# | 16.900 | 132 | 249 | 105 | 458 |
| Fraction 3# | 15.392 | 287 | 497 | 428 | 1570 |
| Depolymerized lignin | 17.843 | 371 | 532 | 536 | 2146 |
The highest peak of the three phenolic compound fractions was 428, 154, and 45 at the retention times of about 16, 14 and 16 min, respectively. The molecular weights (Mw) of fractions 1#, 2#, and 3# and depolymerized lignin were 797, 249, 497, and 532, respectively. The Mw of the three compound fractions with single aromatic rings was less than 300 Da, while the phenolic compounds can easily convert into dimers, trimers, tetramers, and other aromatic derivatives, and the unknown compounds in the three compound fractions may have high molecular weights. Therefore, the GPC data reported showed a weight average Mw of the three fractions of more than 200 Da. The molecular weights of fractions 1# and 3# were relatively higher than fraction 2#, which coincides with the GC-MS components and HSQC NMR results. These increasing molecular weights may result in phenol or substituted phenols reacting with aldehydes to form polymers. The three phenolic compound fractions were separated into different molecular weights during the separation process.
3.4. TG-DTG analysis of depolymerized lignin and phenolic compounds
TG distribution against temperature for the three phenolic compound fractions and depolymerized lignin is shown in Fig. 6. The differences in the three phenolic compound fractions and depolymerized lignin were mostly similar to the DTG analysis results. As the temperature increased from 30 °C to 800 °C, both the phenolic compounds and depolymerized lignin followed about four stages. The first stage involved the drying of the samples (from 30 °C to 120 °C). During this stage, the weight of the water in the chemicals was gradually reduced, as were the samples' mass. The second stage involved the warm-up phase pyrolysis of phenolic compounds and depolymerized lignin (from 130 °C to 210 °C). During this stage, the samples began degrading and exhibited a glassy transition. The third stage was the main pyrolysis stage (from 210 °C to 400 °C), in which both the phenolic compounds and depolymerized lignin were converted into small gas molecules and macromolecular volatile solids, causing most of the weight lost. At the third stage, the weight loss of phenolic compounds fractions 2# and 3# was more obvious than fraction 1# and depolymerized lignin. The weight-loss rate of depolymerized lignin reached a maximum of −1.91% min−1 at 278.0 °C; the weight-loss rate of fraction 1# reached a maximum of −1.92% min−1 at 361.7 °C; the weight-loss rate of fraction 2# reached a maximum of −5.29% min−1 at 253.0 °C; and the weight-loss rate of fraction 3# reached a maximum of −3.08% min−1 at 253.9 °C. The fourth stage involved the fiber carbonization of samples (from 400 °C to 800 °C), in which there was an exothermic peak corresponding to the slow pyrolysis of remaining material in the high temperature decomposition process. In this stage, the pyrolysis residual percentage of the three phenolic compound fractions and depolymerized lignin were 38.39% (797.5 °C), 9.58% (797.7 °C), 24.26% (797.5 °C), and 51.83% (797.5 °C), respectively. The residues mainly constituted carbon residue and ash. In general, in the gradual heating-up pyrolysis process (30–800 °C), the mostly phenolic compounds with small molecules in fractions 2# and 3# were pyrolysized into volatile substances. On the other hand, only half the content of the phenols in depolymerized lignin had been pyrolysized into volatile solid and gas.3.5. GC-MS results of depolymerized lignin and phenolic compounds
The GC-MS results of the three phenolic compound fractions and depolymerized lignin are shown in Fig. 7. In this investigation, the main components of the three phenolic compound fractions from liquefied waste mason pine were investigated in detail and are shown in Table S1.† Various phenols and their derivatives were detected in the phenolic product, such as guaiacol, 2-methoxy-4-propyl phenol, 4-ethyl-2-methoxyphenol, 4-ethylguaiacol, and 3-methylcatechol. The percentages of various phenols and their derivatives in three phenolic compounds fractions and depolymerized lignin were 77.59 wt%, 81.76 wt%, 80.19 wt%, and 78.86 wt%, respectively. This result indicates the polymeric lignin in mason pine can be effectively depolymerized into low molecule phenolic products during the liquefaction process. The liquefaction of mason pine not only depolymerized cellulose and hemicellulose, but also decomposed high molecular polymeric lignin into low molecular phenolic compounds, which reactive intermediated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, –OH, and –C
C, –OH, and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. The decomposition of natural lignin structure in biomass occurs by cleaving the dominant linkages including the β-O-4′ (A), β–β (B), and β-5 (C) units.
O groups. The decomposition of natural lignin structure in biomass occurs by cleaving the dominant linkages including the β-O-4′ (A), β–β (B), and β-5 (C) units.
The three phenolic compound fractions included minor cyclohexane and major phenolic monomers, dimers with various functionalities, especially methoxyl, ester, methyl, and other alkyl groups. The components of the three phenolic compound fractions from liquefied mason pine were significantly different. The contents of phenolic compounds fraction 1# with high molecular weights, complex molecular structures and group functions, were mainly 4-propyl cyclohexane-phenol, 3,5-dimethoxy-4-methylphenol, 4-methyl-2,6-diisopropyl phenol, 4-hydroxy-3-methoxyphenyl-2-ethanol, and 2-methoxy-4-propyl phenol. The phenolic compounds fraction 3# with lower molecular weights were mainly 4-acetylguaical, 4-ethyl-2-methoxyphenol, 4-ethylguaiacol, and 3-methylcatechol. The phenolic compounds fraction 2# were mainly phenol, 2-methylphenol, guaiacol, 4-ethyl-2-methoxyphenol, and 4-ethylguaiacol; these phenols have simple molecular structures and bond functionalities. There were only single-aromatic-ring units (such as G and PB units) in phenolic compounds fraction 2#, which were consistent with the result of 2D HSQC spectra. There were about 10 wt% unknown compounds in the GC-MS spectra, which could potentially be phenolic dimers, trimers, tetramers and other aromatic derivatives with complex structures. Absolute structural identification of these complex compounds was not possible to establish, since their authentic standards were not available in GC-MS.
4. Conclusion
Three renewable phenolic compound fractions (fraction 1#, 2#, 3#) were characterized by 2D HSQC NMR techniques. The three phenolic compound fractions were produced from a liquefied waste mason pine and separated from liquefied-oil by water–organic solvent extraction. PB, G, and S units with minor amounts of H aromatics were the basic units of phenolic compounds. The natural lignin A, B, and C units were not present in the phenolic compounds, indicating the depolymerization of lignin occurs during the liquefied biomass process. Besides, there were only single-aromatic-ring units (such as G and PB units) from the HSQC cross-signals of phenolic compound fraction 2#. From the GPC results, the molecular weights of three phenolic compound fractions were significantly different (797, 249, and 497) along with fractions 1#, 2#, and 3#. The pyrolytic lignin could be separated into a high (fraction 1#), lower (fraction 3#), and lowest (fraction 2#) molecular weights. As evidenced by GC-MS analysis, the three phenolic compound fraction products and depolymerized lignin were mainly composed of phenolic derivatives such as 3-methyl-4-ethylphenol, 4-ethyl-2-methoxyphenol, and 3-methylcatechol. The percentages of total phenols and derivatives in three phenolic compound fractions and depolymerized lignin were 77.59 wt%, 81.76 wt%, 80.19 wt%, and 78.86 wt%, respectively. Therefore, the three phenolic compound fractions were clearly quantified and analyzed, so they can be used as high-added-value chemical products.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (31530010 and 31422013). The authors also thank the Research Grant of Jiangsu Province Biomass Energy and Material Key Laboratory (JSBEM-S-201502) for this investigation.Notes and references
- J. Q. Bond, A. A. Upadhye, H. Olcay, G. A. TompsettJae, J. Jae, R. Xing and A. Foster, Energy Environ. Sci., 2014, 7, 1500–1523 CAS.
- A. Limayem and S. Ricke, Prog. Energy Combust. Sci., 2012, 38, 449–467 CrossRef CAS.
- C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695–699 CrossRef CAS PubMed.
- P. Burguete, A. Corma, M. Hitzl, R. Modrego, E. Ponce and M. Renz, Green Chem., 2016, 18, 1051–1060 RSC.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis and P. Langan, Science, 2014, 344, 1246843 CrossRef PubMed.
- Y. Zeng, S. Zhao, S. Yang and S. Y. Ding, Curr. Opin. Biotechnol., 2014, 27, 38–45 CrossRef CAS PubMed.
- F. P. Bouxin, A. McVeigh, F. Tran, N. J. Westwood, M. C. Jarvis and S. D. Jackson, Green Chem., 2015, 17, 1235–1242 RSC.
- Q. Song, F. Wang, J. Cai, Y. Wang, J. Zhang, W. Yu and J. Xu, Energy Environ. Sci., 2013, 6, 994–1007 CAS.
- G. T. Neumann, B. R. Pimentel, D. J. Rensel and J. C. Hicks, Catal. Sci. Technol., 2014, 4, 3953–3963 CAS.
- J. E. Holladay, J. F. White, J. J. Bozell and D. Johnson, Top value-added chemicals from biomass-volume II—results of screening for potential candidates from biorefinery lignin, Pacific Northwest National Laboratory (PNNL), Richland, WA (US), 2007 Search PubMed.
- P. Azadi, O. R. Inderwildi, R. Farnood and D. A. King, Renewable Sustainable Energy Rev., 2013, 21, 506–523 CrossRef CAS.
- P. J. De Wild, W. J. J. Huijgen and R. J. A. Gosselink, Biofuels, Bioprod. Biorefin., 2014, 8, 645–657 CrossRef CAS.
- J. C. Serrano-Ruiz and J. A. Dumesic, Energy Environ. Sci., 2011, 4, 83–99 CAS.
- W. Chen, D. J. McClelland, A. Azarpira, J. Ralph, Z. Luo and G. W. Huber, Green Chem., 2016, 18, 271–281 RSC.
- A. Sanna, T. P. Vispute and G. W. Huber, Appl. Catal., B, 2015, 165, 446–456 CrossRef CAS.
- M. Garcia-Perez, A. Chaala, H. Pakdel, D. Kretschmer and C. Roy, Biomass Bioenergy, 2007, 31, 222–242 CrossRef CAS.
- M. R. Rover, P. H. Hall, P. A. Johnston, R. G. Smith and R. C. Brown, Fuel, 2015, 153, 224–230 CrossRef CAS.
- G. Warner, T. S. Hansen, A. Riisager, E. S. Beach, K. Barta and P. T. Anastas, Bioresour. Technol., 2014, 161, 78–83 CrossRef CAS PubMed.
- H. Ben and A. J. Ragauskas, ChemSusChem, 2012, 5, 1687–1693 CrossRef CAS PubMed.
- J. L. Wen, S. L. Sun, B. L. Xue and R. C. Sun, Materials, 2013, 6, 359–391 CrossRef CAS.
- H. Kim and J. Ralph, Org. Biomol. Chem., 2010, 8, 576–591 CAS.
- J. C. del Rio, J. Rencoret, G. Marques, A. Gutierrez, D. Ibarra, J. I. Santos, J. Jimenez-Barbero, L. M. Zhang and A. T. Martinez, J. Agric. Food Chem., 2008, 56, 9525–9534 CrossRef CAS PubMed.
- J. C. del Rio, J. Rencoret, G. Marques, J. B. Li, G. Gellerstedt, J. Jimenez-Barbero, A. T. Martinez and A. Gutierrez, J. Agric. Food Chem., 2009, 57, 10271–10281 CrossRef CAS PubMed.
- J. J. Villaverde, J. B. Li, M. Ek, P. Ligero and A. de Vega, J. Agric. Food Chem., 2009, 57, 6262–6270 CrossRef CAS PubMed.
- F. C. Lu and J. Ralph, Plant J., 2003, 35, 535–544 CrossRef CAS PubMed.
- F. C. Lu, J. Ralph, K. Morreel, E. Messens and W. Boerjan, Org. Biomol. Chem., 2004, 2, 2888–2890 CAS.
- J. Rencoret, G. Marques, A. Gutierrez, L. Nieto, J. JimenezBarbero, A. T. Martinez and J. C. del Rio, Ind. Crops Prod., 2009, 30, 137–143 CrossRef CAS.
- T. Q. Yuan, S. N. Sun, F. Xu and R. C. Sun, J. Agric. Food Chem., 2011, 59, 10604–10614 CrossRef CAS PubMed.
- J. C. del Rio, J. Rencoret, P. Prinsen, A. T. Martinez, J. Ralph and A. Gutierrez, J. Agric. Food Chem., 2012, 60, 5922–5935 CrossRef CAS PubMed.
- M. P. Pandey and C. S. Kim, Chem. Eng. Technol., 2011, 34, 29–41 CrossRef CAS.
- W. Chen, D. J. McClelland, A. Azarpira, J. Ralph, Z. Luo and G. W. Huber, Green Chem., 2016, 18, 271–281 RSC.
- S. Chu, A. V. Subrahmanyam and G. W. Huber, Green Chem., 2013, 15, 125–136 RSC.
- P. R. Patwardhan, R. C. Brown and B. H. Shanks, ChemSusChem, 2011, 4, 1629–1636 CrossRef CAS PubMed.
- R. Bayerbach, V. D. Nguyen, U. Schurr and D. Meier, J. Anal. Appl. Pyrolysis, 2006, 77, 95–101 CrossRef CAS.
- R. Bayerbach and D. Meier, J. Anal. Appl. Pyrolysis, 2009, 85, 98–100 CrossRef CAS.
- D. Meier, B. van de Beld, A. V. Bridgwater, D. C. Elliott, A. Oasmaa and F. Preto, Renewable Sustainable Energy Rev., 2013, 20, 619–641 CrossRef.
- M. R. Rover, P. H. Hall, P. A. Johnston, R. G. Smith and R. C. Brown, Fuel, 2015, 153, 224–230 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16916c |
| This journal is © The Royal Society of Chemistry 2016 |






